Details of the Drug Combinations
General Information of This Drug (ID: DM20R5F)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
spiramycin; 8025-81-8; ST075006; Spiramycinum; Spiramycine; Provamycin; Sequamycin; Espiramicin; Rovamycin; Antibiotic 799; NSC-64393; RP 5337; Rovamycine; Prestwick_121; 5337 R.P.; Prestwick2_000745; Prestwick3_000745; AC1O4WG0; Spiramycin antibiotic complex; BPBio1_000804; SCHEMBL5032756; AKOS015896378; K430; SR-01000872632; SR-01000872632-1; I06-1973; Spiramycin, European Pharmacopoeia (EP) Reference Standard; Spiramycin from Streptomyces sp., VETRANAL(TM), analytical standard; Spiramycin, Pharmaceutical Secondary Standard
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
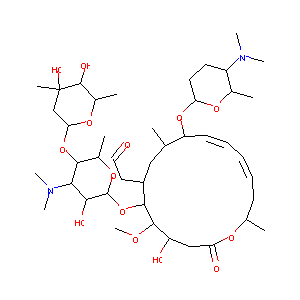 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
