Details of the Drug
General Information of Drug (ID: DM20R5F)
| Drug Name |
Spiramycin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
spiramycin; 8025-81-8; ST075006; Spiramycinum; Spiramycine; Provamycin; Sequamycin; Espiramicin; Rovamycin; Antibiotic 799; NSC-64393; RP 5337; Rovamycine; Prestwick_121; 5337 R.P.; Prestwick2_000745; Prestwick3_000745; AC1O4WG0; Spiramycin antibiotic complex; BPBio1_000804; SCHEMBL5032756; AKOS015896378; K430; SR-01000872632; SR-01000872632-1; I06-1973; Spiramycin, European Pharmacopoeia (EP) Reference Standard; Spiramycin from Streptomyces sp., VETRANAL(TM), analytical standard; Spiramycin, Pharmaceutical Secondary Standard
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
BacteriaStreptococcus pyogenesCorynebacterium diphtheriaeHaemophilus influenzaeStreptococcus viridansBacteria and protozoa
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
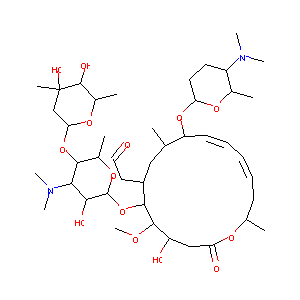 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 843.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 16 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
