Details of the Drug Combinations
General Information of This Drug (ID: DM2FGRT)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
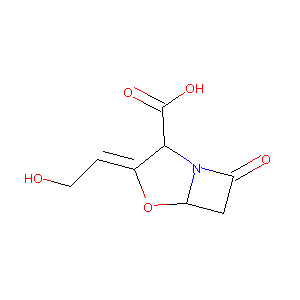
Clavulansaeure; Clavulox; Acide clavulanique; Acido clavulanico; Acidum clavulanicum; CLAVULANIC ACID; Clavulansaeure [INN]; Clavulinic Acid; Sodium Clavulanate; Antibiotic MM 14151; MM 14151; Acide clavulanique [INN-French]; Acido clavulanico [INN-Spanish]; Acidum clavulanicum [INN-Latin]; BRL-14151; Clavulanic acid (INN); Clavulanic acid [BAN:INN]; Clavulox (TN); MM-14151; (2R,3Z,5R)-3-(2-hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (Z)-(2R,5R)-3-(2-Hydroxyethyliden)-7-oxo-4-oxa-1-azabicyclo(3.2.0)heptan-2-carbonsaeure; (Z)-(2R,5R)-3-(2-Hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo(3.2.0)heptane-2-carboxylic acid; 3008-B; 4-Oxa-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 3-(2-hydroxyethylidene)-7-oxo-, (2R-(2alpha,3Z,5alpha))
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
