Details of the Drug Combinations
General Information of This Drug (ID: DM2HKC4)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cortilet; Cortisdin; Delmeson; Efflumidex; FML; Fluaton; Flucon; Flumetholon; Fluormetholon; Fluormetholone; Fluormetholonum; Fluoromethalone; Fluorometholonum; Fluorometolona; Fluorometolone; Fluoropos; Loticort; Oxylone; Trilcin; Ursnon; Alcon Brand of Fluorometholone; FML Forte; FML Liquifilm; Fluor Op; Fluoro Ophtal; Fluorometolone [DCIT]; Isdin Brand of Fluorometholone; Isopto Flucon; Novartis Brand of Fluorometholone; PMS Fluorometholone; Pharm Allergan Brand of Fluorometholone; Pharmascience Brand of Fluorometholone; Ursapharm Brand of Fluorometholone; Winzer Brand of Fluorometholone; Allergan Brand 1 of Fluorometholone; Allergan Brand 2 of Fluorometholone; Allergan Brand 3 of Fluorometholone; F0414; U 8614; Component of Neo-Oxylone; FML (TN); FML-S Liquifilm; Flarex (TN); Flucon, Isopto; Fluor-Op; Fluoro-Ophtal; Fluorometholonum [INN-Latin]; Fluorometolona [INN-Spanish]; Fml (TN); Neo-Oxylone; Oxylone (TN); PMS-Fluorometholone; Pharm-Allergan Brand of Fluorometholone; FML S.O.P; Fluor-op (TN); Fluorometholone [INN:BAN:JAN]; Fluorometholone (JP15/USP/INN); Pregna-1,4-diene-3,20-dione, 9-fluoro-11.beta.,17; Pregna-1,4-diene-3,20-dione, 9-fluoro-11beta,17-dihydroxy-6alpha-methyl-(8CI); (6S,8S,9R,10S,11S,13S,14S,17R)-17-acetyl-9-fluoro-11,17-dihydroxy-6,10,13-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one; 11beta,17alpha-Dihydroxy-9-fluoro-6-methyl-1,4-pregnadiene-3,20-dione; 9-Fluoro-11,17-dihydroxy-6-methylpregna-1,4-diene-3,20-dione; 9-Fluoro-11-beta,17-dihydroxy-6-alpha-methylpregna-1,4-diene-3,20-dione; 9-Fluoro-11beta,17-dihydroxy-6alpha-methylpregna-1,4-diene-3,20-dione
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiallergic Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
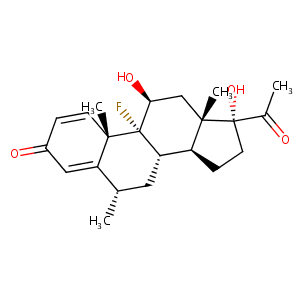 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
