Details of the Drug
General Information of Drug (ID: DM2HKC4)
| Drug Name |
Fluorometholone
|
|||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cortilet; Cortisdin; Delmeson; Efflumidex; FML; Fluaton; Flucon; Flumetholon; Fluormetholon; Fluormetholone; Fluormetholonum; Fluoromethalone; Fluorometholonum; Fluorometolona; Fluorometolone; Fluoropos; Loticort; Oxylone; Trilcin; Ursnon; Alcon Brand of Fluorometholone; FML Forte; FML Liquifilm; Fluor Op; Fluoro Ophtal; Fluorometolone [DCIT]; Isdin Brand of Fluorometholone; Isopto Flucon; Novartis Brand of Fluorometholone; PMS Fluorometholone; Pharm Allergan Brand of Fluorometholone; Pharmascience Brand of Fluorometholone; Ursapharm Brand of Fluorometholone; Winzer Brand of Fluorometholone; Allergan Brand 1 of Fluorometholone; Allergan Brand 2 of Fluorometholone; Allergan Brand 3 of Fluorometholone; F0414; U 8614; Component of Neo-Oxylone; FML (TN); FML-S Liquifilm; Flarex (TN); Flucon, Isopto; Fluor-Op; Fluoro-Ophtal; Fluorometholonum [INN-Latin]; Fluorometolona [INN-Spanish]; Fml (TN); Neo-Oxylone; Oxylone (TN); PMS-Fluorometholone; Pharm-Allergan Brand of Fluorometholone; FML S.O.P; Fluor-op (TN); Fluorometholone [INN:BAN:JAN]; Fluorometholone (JP15/USP/INN); Pregna-1,4-diene-3,20-dione, 9-fluoro-11.beta.,17; Pregna-1,4-diene-3,20-dione, 9-fluoro-11beta,17-dihydroxy-6alpha-methyl-(8CI); (6S,8S,9R,10S,11S,13S,14S,17R)-17-acetyl-9-fluoro-11,17-dihydroxy-6,10,13-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one; 11beta,17alpha-Dihydroxy-9-fluoro-6-methyl-1,4-pregnadiene-3,20-dione; 9-Fluoro-11,17-dihydroxy-6-methylpregna-1,4-diene-3,20-dione; 9-Fluoro-11-beta,17-dihydroxy-6-alpha-methylpregna-1,4-diene-3,20-dione; 9-Fluoro-11beta,17-dihydroxy-6alpha-methylpregna-1,4-diene-3,20-dione
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiallergic Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
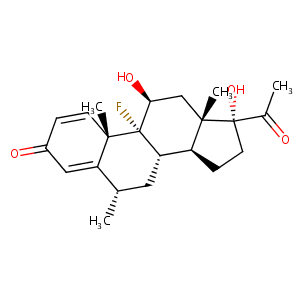 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 376.5 | ||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | |||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic obstructive pulmonary disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA22 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Fluorometholone (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | Fluorometholone FDA Label | ||||
| 3 | Loteprednol etabonate: a soft steroid for the treatment of allergic diseases of the airways. Drugs Today (Barc). 2000 May;36(5):313-20. | ||||
| 4 | Regulation of drug-metabolizing cytochrome P450 enzymes by glucocorticoids. Drug Metab Rev. 2010 Nov;42(4):621-35. | ||||
| 5 | Edsbacker S, Andersson T "Pharmacokinetics of budesonide (Entocort EC) capsules for Crohn's disease." Clin Pharmacokinet 43 (2004): 803-21. [PMID: 15355126] | ||||
