Details of the Drug Combinations
General Information of This Drug (ID: DM2M3ZA)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
GLYCOPYRROLATE; 596-51-0; Glycopyrrolate bromide; Robinul; Gastrodyn; Tarodyl; Nodapton; Tarodyn; Asecryl; Copyrrolate; Cuvposa; Glycopyrronii bromidum; AHR-504; ROBINUL FORTE; Robinal; Robanul; Bromuro de glicopirronio; Bromure de glycopyrronium; NVA-237; AHR 504; Glycopyrronii bromidum [INN-Latin]; EINECS 209-887-0; Bromure de glycopyrronium [INN-French]; Bromuro de glicopirronio [INN-Spanish]; 3-Hydroxy-1,1-dimethylpyrrolidinium bromide
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Anticholinergic Agents
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
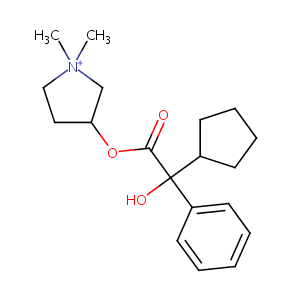 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
11 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
1 Approved Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
