| 1 |
ClinicalTrials.gov (NCT02574611) Use of High Resolution Colonic Manometry in Studying Motility
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7459).
|
| 3 |
Clinical pipeline report, company report or official report of Novartis.
|
| 4 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 5 |
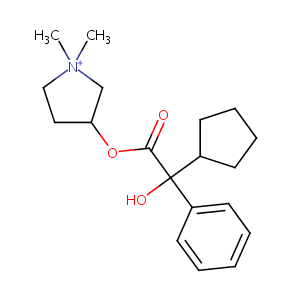
Neostigmine FDA Label
|
| 6 |
Autonomic cardiovascular control during a novel pharmacologic alternative to ganglionic blockade. Clin Pharmacol Ther. 2008 May;83(5):692-701.
|
| 7 |
Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42.
|
| 8 |
Screening of acetylcholinesterase inhibitors by CE after enzymatic reaction at capillary inlet. J Sep Sci. 2009 May;32(10):1748-56.
|
| 9 |
Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev. 2012 Jan;64(1):95-109.
|
| 10 |
Hairy-root organ cultures for the production of human acetylcholinesterase. BMC Biotechnol. 2008 Dec 23;8:95.
|
| 11 |
Fresh frozen plasma transfusion for reversal of prolonged post-anaesthesia apnoea. Transfus Med. 2008 Apr;18(2):134-6. doi: 10.1111/j.1365-3148.2008.00851.x.
|
| 12 |
ClinicalTrials.gov (NCT01199237) Impact of Anesthetic Choice (Sevoflurane Versus Desflurane) on Airway Reflex Recovery in the Context of Antagonized Neuromuscular Block
|
| 13 |
ClinicalTrials.gov (NCT03939923) Role of Sugammadex as Reversal Agent in Patients Extubated Immediately After Isolated Coronary Artery Bypass Grafting Surgery
|
| 14 |
ClinicalTrials.gov (NCT00473694) Comparison of Sugammadex (MK-8616/Org 25969) With Neostigmine Administered at 1-2 Post-tetanic Counts (PTCs) After Administration of Rocuronium or Vecuronium (19.4.302/P05945/MK-8616-025)
|
| 15 |
ClinicalTrials.gov (NCT01479764) Study of Sugammadex Versus Usual Care on Incidence of Residual Blockade at Post Anesthesia Care Unit Admission (P07981)
|
|
|
|
|
|
|