Details of the Drug Combinations
General Information of This Drug (ID: DM3NMT6)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Articaine; Carticaine; 23964-58-1; Articaine [INN:BAN]; Articainum [INN-Latin]; Articaina [INN-Spanish]; Hoe-045; Ultracain; HOE 045; methyl 4-methyl-3-[(N-propylalanyl)amino]thiophene-2-carboxylate; methyl 4-methyl-3-[2-(propylamino)propanoylamino]thiophene-2-carboxylate; Methyl (4-methyl-3-(2-(propylamino)propionamido)-2-thiophencarboxylat); Q-200652; Articainum; Articaina; 4-Methyl-3-(2-(propylamino)propionamido)-2-thiophenecarboxylic acid, methyl ester; 2-Thiophenecarboxylicacid, 4-methyl-3-[[1-oxo-2-(propylamino)propy
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
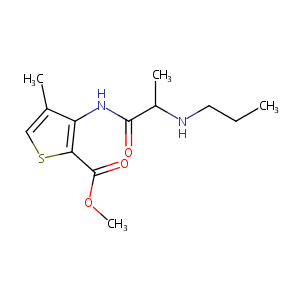 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
