Details of the Drug Combinations
General Information of This Drug (ID: DM3UQ9K)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acolbifene; EM-652; 182167-02-8; UNII-815LJ9X0D1; 815LJ9X0D1; EM 652; Acolbifene [INN:BAN]; SCH 57068; AC1L4EAA; SCHEMBL406183; CHEMBL68055; CTK4F5320; SCH-57068; HY-16023A; ( )-(2S)-3-(4-Hydroxyphenyl)-4-methyl-2-(4-(2-(piperidin-1-yl)ethoxy)phenyl)-2H-1-benzopyran-7-ol; CS-0007143; (2S)-3-(4-hydroxyphenyl)-4-methyl-2-[4-(2-piperidin-1-ylethoxy)phenyl]-2H-chromen-7-ol; (S)-3-(4-hydroxyphenyl)-4-methyl-2-(4-(2-(piperidin-1-yl)ethoxy)phenyl)-2H-chromen-7-ol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
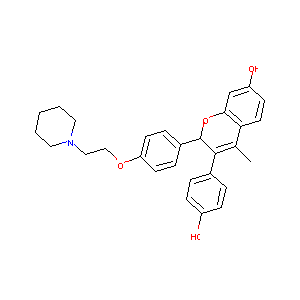 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
References
