Details of the Drug Combinations
General Information of This Drug (ID: DM3XNUI)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
TAZ; CL298741; YTR830H; CL-298741; Piprataz (TN); YTR-830H; Tazobactam (JAN/USAN/INN); (2S,3S,5R)-3-methyl-4,4,7-trioxo-3-(triazol-1-ylmethyl)-4; (2S,3S,5R)-3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiinfective Agents
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
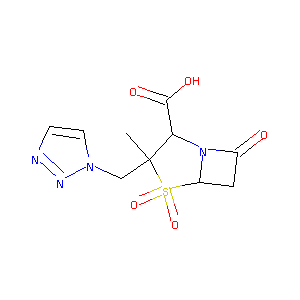 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
