Details of the Drug Combinations
General Information of This Drug (ID: DM3XRUH)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tropifexor; 1383816-29-2; UNII-NMZ08KM76Z; NMZ08KM76Z; CPD1549; Tropifexor [INN]; GTPL9725; SCHEMBL19178329; SCHEMBL17848159; LJN-452; EX-A1934; CS-8153; compound 1 [PMID: 29148806]; ACN-053193; AC-30341; 2-[(1R,3r,5S)-3-({5-cyclopropyl-3-[2-(trifluoromethoxy)phenyl]-1,2-oxazol-4-yl}methoxy)-8-azabicyclo[3.2.1]octan-8-yl]-4-fluoro-1,3-benzothiazole-6-carboxylic acid; HY-107418; 2-((1R,5S)-3-((5-cyclopropyl-3-(2-(trifluoromethoxy)phenyl)isoxazol-4-yl)methoxy)-8-azabicyclo[3.2.1]octan-8-yl)-4-fluorob
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
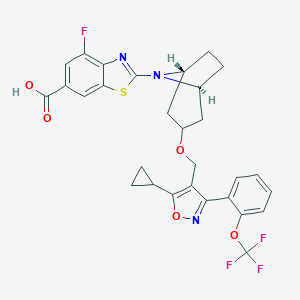 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
