Details of the Drug Combinations
General Information of This Drug (ID: DM4N7BT)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
(2R,6S,13aS,14aR,16aS,Z)-N-(cyclopropylsulfonyl)-6-(5-methylpyrazine-2-carboxamido)-5,16-dioxo-2-(phenanthridin-6-yloxy)-1,2,3,5,6,7,8,9,10,11,13a,14,14a,15,16,16a-hexadecahydrocyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a-carboxamide; ABT 450; ABT-450; ABT450; CHEMBL3391662; EX-A2278; OU2YM37K86; Paritaprevir; Paritaprevir [USAN:INN]; Paritaprevir(ABT-450); Paritaprevir(Veruprevir ABT-450); SCHEMBL3069964; UNII-OU2YM37K86; Veruprevir; Veruprevir [INN]; Veruprevir anhydrous
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiviral Agent
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
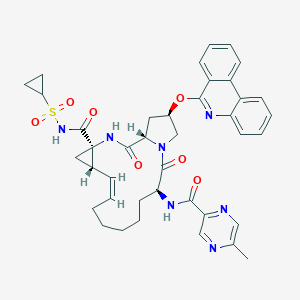 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
|
1 Approved Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
