Details of the Drug Combinations
General Information of This Drug (ID: DM6034S)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
selegiline; L-Deprenalin; Emsam; (-)-selegiline; Selegilinum; Selegilina; Carbex; 14611-51-9; Selegilinum [INN-Latin]; Selegilina [INN-Spanish]; UNII-2K1V7GP655; l-E 250; CHEMBL972; CHEBI:9086; N-methyl-N-[(2R)-1-phenylpropan-2-yl]prop-2-yn-1-amine; 2K1V7GP655; (R)-(-)-N,alpha-Dimethyl-N-2-propinylphenethylamine; Benzeneethanamine, N,alpha-dimethyl-N-2-propynyl-, (R)-; selgene; (R)-(-)-N-Methyl-N-(1-phenyl-2-propyl)-2-propinylamin; Selegyline; Zalapar; Selegiline (transdermal, Parkinson's/depression); Zunrisa/Rezonic
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
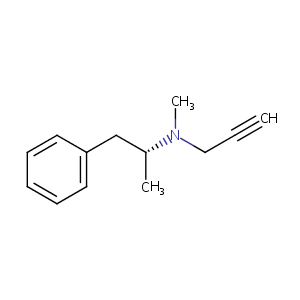 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
