Details of the Drug
General Information of Drug (ID: DM6034S)
| Drug Name |
Selegiline
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
selegiline; L-Deprenalin; Emsam; (-)-selegiline; Selegilinum; Selegilina; Carbex; 14611-51-9; Selegilinum [INN-Latin]; Selegilina [INN-Spanish]; UNII-2K1V7GP655; l-E 250; CHEMBL972; CHEBI:9086; N-methyl-N-[(2R)-1-phenylpropan-2-yl]prop-2-yn-1-amine; 2K1V7GP655; (R)-(-)-N,alpha-Dimethyl-N-2-propinylphenethylamine; Benzeneethanamine, N,alpha-dimethyl-N-2-propynyl-, (R)-; selgene; (R)-(-)-N-Methyl-N-(1-phenyl-2-propyl)-2-propinylamin; Selegyline; Zalapar; Selegiline (transdermal, Parkinson's/depression); Zunrisa/Rezonic
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
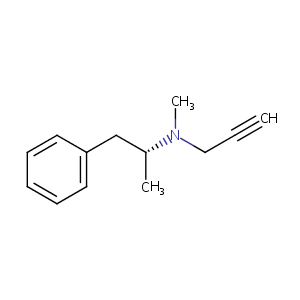 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 187.28 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Major depressive disorder | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A70.3 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Selegiline
Coadministration of a Drug Treating the Disease Different from Selegiline (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6639). | ||||
|---|---|---|---|---|---|
| 2 | Novel monoamine oxidase inhibitors: a patent review (2012 - 2014).Expert Opin Ther Pat. 2015 Jan;25(1):91-110. | ||||
| 3 | Clinical pipeline report, company report or official report of GlaxoSmithKline. | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2490). | ||||
| 8 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | ||||
| 9 | Characterizing fucoxanthin as a selective dopamine D(3)/D(4) receptor agonist: Relevance to Parkinson's disease. Chem Biol Interact. 2019 Sep 1;310:108757. doi: 10.1016/j.cbi.2019.108757. Epub 2019 Jul 16. | ||||
| 10 | Chromone, a privileged scaffold for the development of monoamine oxidase inhibitors. J Med Chem. 2011 Jul 28;54(14):5165-73. | ||||
| 11 | Oral contraceptives containing ethinyl estradiol and gestodene markedly increase plasma concentrations and effects of tizanidine by inhibiting cytochrome P450 1A2. Clin Pharmacol Ther. 2005 Oct;78(4):400-11. | ||||
| 12 | Evaluation of metabolism dependent inhibition of CYP2B6 mediated bupropion hydroxylation in human liver microsomes by monoamine oxidase inhibitors and prediction of potential as perpetrators of drug interaction. Chem Biol Interact. 2015 Mar 25;230:9-20. | ||||
| 13 | Role of the redox protein thioredoxin in cytoprotective mechanism evoked by (-)-deprenyl. Mol Pharmacol. 2005 Nov;68(5):1408-14. doi: 10.1124/mol.105.012302. Epub 2005 Aug 12. | ||||
| 14 | Beasley CM Jr, Masica DN, Heiligenstein JH, Wheadon DE, Zerbe RL "Possible monoamine oxidase inhibitor-serotonin uptake inhibitor interaction: fluoxetine clinical data and preclinical findings." J Clin Psychopharmacol 13 (1993): 312-20. [PMID: 8227489] | ||||
| 15 | Alvine G, Black DW, Tsuang D "Case of delirium secondary to phenelzine/L-tryptophan combination." J Clin Psychiatry 51 (1990): 311. [PMID: 2365671] | ||||
| 16 | Boyer EW, Shannon M "The serotonin syndrome." N Engl J Med 352 (2005): 1112-20. [PMID: 15784664] | ||||
| 17 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 18 | Abad S, Moachon L, Blanche P, Bavoux F, Sicard D, Salmon-Ceron D "Possible interaction between glicazide, fluconazole and sulfamethoxazole resulting in severe hypoglycaemia." Br J Clin Pharmacol 52 (2001): 456-7. [PMID: 11678792] | ||||
| 19 | Asplund K, Wiholm BE, Lithner F "Glibenclamide-associated hypoglycaemia: a report on 57 cases." Diabetologia 24 (1983): 412-7. [PMID: 6411511] | ||||
| 20 | Product Information. Marplan (isocarboxazid) Roche Laboratories, Nutley, NJ. | ||||
| 21 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 22 | Boakes AJ, Laurence DR, Teoh PC, Barar FS, Benedikter LT, Prichard BN "Interactions between sympathomimetic amines and antidepressant agents in man." Br Med J 1 (1973): 311-5. [PMID: 4685619] | ||||
| 23 | Adverse effects and complications of treatment with beta-adrenergic agonist drugs. Committee on drugs, the American Academy of Allergy and Immunology. J Allergy Clin Immunol 75 (1985): 443-9. [PMID: 2858503] | ||||
| 24 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 25 | Laine K, Anttila M, Helminen A, Karnani H, Huupponen R "Dose linearity study of selegiline pharmacokinetics after oral administration: evidence for strong drug interaction with female sex steroids." Br J Clin Pharmacol 47 (1999): 249-54. [PMID: 10215747] | ||||
| 26 | Cerner Multum, Inc. "Canadian Product Information.". | ||||
| 27 | Graber MA, Hoehns TB, Perry PJ "Sertraline-phenelzine drug interaction: a serotonin syndrome reaction." Ann Pharmacother 28 (1994): 732-5. [PMID: 7919561] | ||||
| 28 | Goldberg LI "Monoamine oxidase inhibitors: adverse reactions and possible mechanisms." JAMA 190 (1964): 456-62. [PMID: 14197995] | ||||
| 29 | Product Information. Austedo (deutetrabenazine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 30 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 31 | Darcy PF, Griffin JP "Interactions with drugs used in the treatment of depressive illness." Adverse Drug React Toxicol Rev 14 (1995): 211-31. [PMID: 8845455] | ||||
| 32 | Cusson JR, Goldenberg E, Larochelle P "Effect of a novel monoamine-oxidase inhibitor, moclobemide on the sensitivity to intravenous tyramine and norepinephrine in humans." J Clin Pharmacol 31 (1991): 462-7. [PMID: 2050833] | ||||
| 33 | De Vita VT, Hahn MA, Oliverio VT "Monoamine oxidase inhibition by a new carcinostatic agent, n-isopropyl-a-(2-methylhydrazino)-p-toluamide (MIH). (30590)." Proc Soc Exp Biol Med 120 (1965): 561-5. [PMID: 4379192] | ||||
| 34 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 35 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 36 | Filibeck DJ, Grimm D, Forman WB, Leidner BA "Metoclopramide-induced hypertensive crisis." Clin Pharm 3 (1984): 548-9. [PMID: 6541544] | ||||
| 37 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
