Details of the Drug Combinations
General Information of This Drug (ID: DM6DEK9)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
DdeThd; DdeTyd; Dideoxydidehydrothymidine; Estavudina; STV; Sanilvudine; Stavudinum; Zent; Zerit; Zerit Xr; Zerut XR; BMY 27857; BMY27857; D 1413; D 4T; BMY-27857; Bristol-Myers Brand of Stavudine; Bristol-Myers Squibb Brand of Stavudine; D 4T (nucleoside); Estavudina [INN-Spanish]; Sanilvudine (JAN); Stavudine, Monosodium Salt; Stavudinum [INN-Latin]; Zerit (TN); Zerit(TM); D4T & GM-CSF; D4TMBY-27857-3; Stavudine (USAN/INN); Stavudine [USAN:BAN:INN]; Stavudine [USAN:INN:BAN]; Thymine, 1-(2,3-dideoxy-beta-D-glycero-pent-2-enofuranosyl)-(7CI,8CI); 1-(2,3-Dideoxy-beta-D-glycero-2-pentenofuranosyl)thymine; 1-(2,3-Dideoxy-beta-D-glycero-pent-2-enofuranosyl)thymine; 1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methylpyrimidine-2,4(1H,3H)-dione; 1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methylpyrimidine-2,4-dione; 1-[5-(hydroxymethyl)-2,5-dihydro-2-furanyl]-5-methyl-2,4(1H,3H)-pyrimidinedione & Colony-stimulating factor 2; 2',3' Didehydro 3' deoxythymidine; 2',3'-Anhydrothymidine; 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE (DDI); 2',3'-Didehydro-2',3'-dideoxythmidine; 2',3'-Didehydro-3'-deoxythimidine; 2',3'-Didehydro-3'-deoxythymidine; 3'-Azido-3'-deoxythymidine & Granulocyte-macrophage colony-stimulating factor; 3'-Deoxy-2',3'-didehydrothymidine; 3'-Deoxy-2'-thymidinene; D4T
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
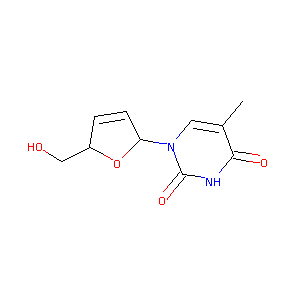 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||
|
4 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||
References
