Details of the Drug
General Information of Drug (ID: DM6DEK9)
| Drug Name |
Stavudine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
DdeThd; DdeTyd; Dideoxydidehydrothymidine; Estavudina; STV; Sanilvudine; Stavudinum; Zent; Zerit; Zerit Xr; Zerut XR; BMY 27857; BMY27857; D 1413; D 4T; BMY-27857; Bristol-Myers Brand of Stavudine; Bristol-Myers Squibb Brand of Stavudine; D 4T (nucleoside); Estavudina [INN-Spanish]; Sanilvudine (JAN); Stavudine, Monosodium Salt; Stavudinum [INN-Latin]; Zerit (TN); Zerit(TM); D4T & GM-CSF; D4TMBY-27857-3; Stavudine (USAN/INN); Stavudine [USAN:BAN:INN]; Stavudine [USAN:INN:BAN]; Thymine, 1-(2,3-dideoxy-beta-D-glycero-pent-2-enofuranosyl)-(7CI,8CI); 1-(2,3-Dideoxy-beta-D-glycero-2-pentenofuranosyl)thymine; 1-(2,3-Dideoxy-beta-D-glycero-pent-2-enofuranosyl)thymine; 1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methylpyrimidine-2,4(1H,3H)-dione; 1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methylpyrimidine-2,4-dione; 1-[5-(hydroxymethyl)-2,5-dihydro-2-furanyl]-5-methyl-2,4(1H,3H)-pyrimidinedione & Colony-stimulating factor 2; 2',3' Didehydro 3' deoxythymidine; 2',3'-Anhydrothymidine; 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE (DDI); 2',3'-Didehydro-2',3'-dideoxythmidine; 2',3'-Didehydro-3'-deoxythimidine; 2',3'-Didehydro-3'-deoxythymidine; 3'-Azido-3'-deoxythymidine & Granulocyte-macrophage colony-stimulating factor; 3'-Deoxy-2',3'-didehydrothymidine; 3'-Deoxy-2'-thymidinene; D4T
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
||||||||||||||||||||||
| Affected Organisms |
Human Immunodeficiency Virus
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
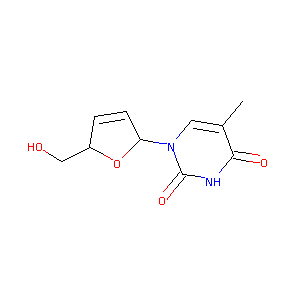 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 224.21 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Stavudine
Coadministration of a Drug Treating the Disease Different from Stavudine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Primer unblocking by HIV-1 reverse transcriptase and resistance to nucleoside RT inhibitors (NRTIs). Int J Biochem Cell Biol. 2004 Sep;36(9):1687-705. | ||||
| 7 | Lon protease and eiF2 are involved in acute, but not prolonged, antiretroviral induced stress response in HepG2 cells. Chem Biol Interact. 2016 May 25;252:82-6. doi: 10.1016/j.cbi.2016.03.021. Epub 2016 Apr 1. | ||||
| 8 | Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther. 2007;12(4):489-500. | ||||
| 9 | Binding of anti-HIV drugs to human serum albumin. IUBMB Life. 2004 Oct;56(10):609-14. doi: 10.1080/15216540400016286. | ||||
| 10 | Stavudine selectively induces apoptosis in HIV type 1-infected cells. AIDS Res Hum Retroviruses. 1997 Jan 20;13(2):193-9. doi: 10.1089/aid.1997.13.193. | ||||
| 11 | Longitudinal effects of thymidine analogues on mtDNA, mtRNA and multidrug resistance (MDR-1) induction in cultured cells. J Antimicrob Chemother. 2008 May;61(5):1048-52. doi: 10.1093/jac/dkn067. Epub 2008 Feb 28. | ||||
| 12 | Mitochondrial proliferation, DNA depletion and adipocyte differentiation in subcutaneous adipose tissue of HIV-positive HAART recipients. Antivir Ther. 2003 Aug;8(4):323-31. | ||||
| 13 | Facilitated mitochondrial import of antiviral and anticancer nucleoside drugs by human equilibrative nucleoside transporter-3. Am J Physiol Gastrointest Liver Physiol. 2009 Apr;296(4):G910-22. doi: 10.1152/ajpgi.90672.2008. Epub 2009 Jan 22. | ||||
| 14 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 15 | Argov Z, Mastaglia FL "Drug-induced peripheral neuropathies." Br Med J 1 (1979): 663-6. [PMID: 219931] | ||||
| 16 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 17 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 18 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 19 | Guyader D, Poinsignon Y, Cano Y, Saout L "Fatal lactic acidosis in a HIV-positive patient treated with interferon and ribavirin for chronic hepatitis C." J Hepatol 37 (2002): 289-91. [PMID: 12127440] | ||||
| 20 | Product Information. Accolate (zafirlukast). Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 21 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 22 | Canadian Pharmacists Association. | ||||
| 23 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 24 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 25 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 26 | MHRA. Medicines and Healthcare Products Regulatory Agency "Orlistat: theoretical interaction with antiretroviral HIV medicines.". | ||||
| 27 | Product Information. ReVia (naltrexone). DuPont Pharmaceuticals, Wilmington, DE. | ||||
