Details of the Drug Combinations
General Information of This Drug (ID: DM6HX9B)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
NEV; NVP; Viramune; Cahill May Roberts Brand of Nevirapine; Promeco Brand of Nevirapine; BI RG 587; BIRG 0587; BIRG 587; BIRG587; BIRG-0587; BIRG-587; NON-NUCLEOSIDE RT INHIBITOR NEVIRAPINE; Nevirapine & PRO 140; Nevirapine [USAN:INN]; Viramune (TN); Viramune(TM); BI-RG-587; Nevirapine & CD4-IgG; Nevirapine (JAN/USP/INN); Viramune, BI-RG 587, Nevirapine; BI-RG-587 & CD4-IgG; N11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[3,2-b:2',3'-e]-[1,4]diazepin-6-one & CD4-immunoadhesin; 11-CYCLOPROPYL-5,11-DIHYDRO-4-METHYL-6H-DIPYRIDO[3,2-B:2',3'-E][1,4]DIAZEPIN-6-ONE; 11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[2,3-e:3',2'-b][1,4]diazepin-6-one; 11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[2,3-e:3',2'-b][1,4]diazepin-6-one & PRO 140 (Anti-CCR5 monoclonal antibody); 11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one; 11-Cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido(3,2-b:2',3'-e)(1,4)diazepin-6-one
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
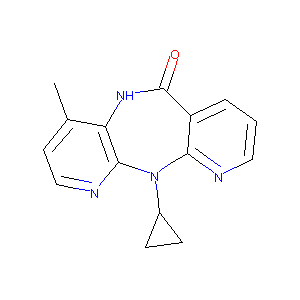 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
11 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
