Details of the Drug Combinations
General Information of This Drug (ID: DM70BU5)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Algosediv; Asmadion; Asmaval; Bonbrain; Bonbrrin; Calmore; Calmorex; Contergan; Corronarobetin; Distaval; Distaxal; Distoval; Ectiluran; Enterosediv; Gastrinide; Glupan; Glutanon; Grippex; Hippuzon; Imidene; Isomin; Kedavon; Kevadon; Neaufatin; Neosedyn; Neosydyn; Nerosedyn; Neufatin; Neurodyn; Neurosedin; Neurosedym; Neurosedyn; Nevrodyn; Nibrol; Noctosediv; Noxodyn; Pangul; Pantosediv; Polygripan; Profarmil; Psycholiquid; Psychotablets; Quetimid; Quietoplex; Sandormin; Sedalis; Sedimide; Sedin; Sedisperil; Sedoval; Shinnibrol; Sleepan; Slipro; Softenil; Softenon; Synovir; Talargan; Talidomida; Talidomide; Talimol; Talismol; Talizer; Telagan; Telargan; Telargean; Tensival; Thaled; Thalidomidum; Thalin; Thalinette; Thalomid; Thalomide; Theophilcholine; Valgis; Valgraine; Yodomin; Celgene Brand of Thalidomide; Talidomide [DCIT]; Thalidomide Celgene; Thalidomide Pharmion; Asidon 3; ENMD 0995; IN1061; Thalidomine USP26; Alpha-Phthalimidoglutarimide; E-217; Imida-lab; Imidan (peyta); N-Phthalimidoglutamic acid imide; N-Phthaloylglutamimide; N-Phthalylglutamic acid imide; Poly-Giron; Predni-Sediv; Pro-Bam M; Pro-ban M; Sedalis sedi-lab; Shin-naito S; THALIDOMIDE (AIDS INITIATIVE); Talidomida [INN-Spanish]; Thaled (TN); Thalidomide (soluble form); Thalidomidum [INN-Latin]; Thalomid (TM); Thalomid (TN); Thalomid, Thalidomide; Alpha-N-Phthalylglutaramide; Thalidomide [USAN:INN:BAN]; Alpha-(N-Phthalimido)glutarimide; N-Phthalyl-glutaminsaeure-imid; N-Phthalyl-glutaminsaeure-imid [German]; Thalidomide (+ and-); Thalidomide (JAN/USP/INN); N-(2,6-Dioxo-3-piperidyl)phthalimide; (+)-Thalidomide; (+-)-Thalidomide; (+/-)-THALIDOMIDE; (inverted question mark)-Thalidomide; 2,6-Dioxo-3-phthalimidopiperidine; 3-Phthalimidoglutarimide
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Immunosuppressive Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
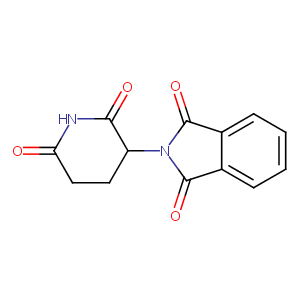 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
