Details of the Drug Combinations
General Information of This Drug (ID: DM7G0EP)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
FOM; Fosmidomycina; Fosmidomycine; Fosmidomycinum; FR-31564; Fosmidomycina [INN-Spanish]; Fosmidomycine [INN-French]; Fosmidomycinum [INN-Latin]; {3-[formyl(hydroxy)amino]propyl}phosphonic acid; (3-(Formylhydroxyamino)propyl)phosphonic acid, monosodium salt; (3-(N-Hydroxyformamido)propyl)phosphonic acid; 3-(Formyl-hydroxy-amino)propylphosphonic acid; 3-(N-Formyl-N-hydroxyamino)-propylphosphonic acid; 3-(N-hydroxyformamido)propylphosphonic acid; 3-[FORMYL(HYDROXY)AMINO]PROPYLPHOSPHONIC ACID
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antimalarials
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
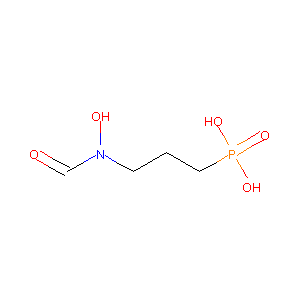 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
