Details of the Drug Combinations
General Information of This Drug (ID: DM8SXYG)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Crisomet; Labileno; Lamictal; Lamictin; Lamiktal; Lamitor; Lamotrigina; Lamotriginum; Desitin Brand of Lamotrigine; Faes Brand of Lamotrigine; Glaxo Wellcome Brand of Lamotrigine; GlaxoSmithKline Brand of Lamotrigine; Juste Brand of Lamotrigine; Lamictal Cd; Lamictal ODT; Lamictal XR; Lamotrigina [Spanish]; Lamotriginum [Latin]; BW 430C; GI 267119X; GW 273293; L 3791; BW-430C; EUR-1048; Lamictal (TN); Lamictin (TN); Lamotrigine [USAN:INN:BAN]; Lamotrigine (JAN/USAN/INN); 3,5-Diamino-6-(2,3-dichlorophenyl)-1,2,4-triazine; 3,5-Diamino-6-(2,3-dichlorophenyl)-as-triazine; 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine; 6-(2,3-Dichlorophenyl)-1,2,4-triazine-3,5-diamine; 6-(2,3-Dichlorophenyl)-1,2,4-triazine-3,5-diyldiamine; 6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diamine
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Anticonvulsants
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
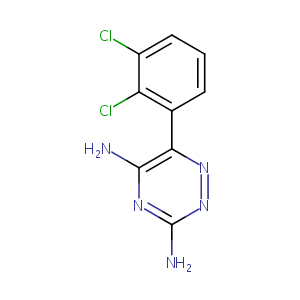 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
10 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
