Details of the Drug Combinations
General Information of This Drug (ID: DM8ZXT6)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Hazol; Iliadin; Nafrine; Navasin; Navisin; Nezeril; Oximetazolina; Oximetazolinum; Oxylazine; Oxymetazolinum; Oxymethazoline; Oxymetozoline; Rhinofrenol; Rhinolitan; Sinerol; Dristan Long Lasting Mentholated Nasal Spray; Dristan Long Lasting Nasal Mist; Drixoral Nasal Solution; Oxymeta zoline; Oxymetazoline hydrochloride crystalline; Afrin Cherry 12 Hour Nasal Spray; Afrin Extra Moisturizing 12 Hour Nasal Spray; Afrin Original 12 Hour Nasal Spray; Afrin Original 12 Hour Nose Drops; Afrin Original 12 Hour Pump Mist; Afrin Sinus 12 Hour Nasal Spray; Duramist Plus Up To 12 Hour Nasal Decongestant Spray; Genasal Nasal Spray Up to 12 Hour Relief; H 990; Nasal Relief 12 Hour Nasal Spray; Nostrilla 12 Hour Nasal Decongestant; Vicks Sinex 12 Hour Nasal Spray; Vicks Sinex 12 Hour Ultra Fine Mist for Sinus Relief; Decongestant (TN); Neo-Synephrine 12 Hour Spray; Operil (TN); Oximetazolina [INN-Spanish]; Oxymetazoline (INN); Oxymetazoline [INN:BAN]; Oxymetazolinum [INN-Latin]; Visine L.R; Visine L.R.; Phenol, 6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethyl-(7CI,8CI); Phenol, 3-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethyl-(9CI); 2-(4-tert-Butyl-2,6-dimethyl-3-hydroxybenzyl)-2-imidazoline; 3-(4,5-dihydro-1H-imidazol-2-ylmethyl)-2,4-dimethyl-6-tert-butyl-phenol; 3-[(4,5-Dihydro-1H-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethylphenol; 6-t-Butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethylphenol; 6-tert-Butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethylphenol; 6-tert-Butyl-3-(4,5-dihydro-1H-imidazol-2-ylmethyl)-2,4-dimethylphenol
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Nasal Decongestants
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
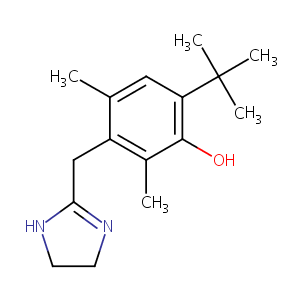 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
