Details of the Drug
General Information of Drug (ID: DM8ZXT6)
| Drug Name |
Oxymetazoline
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Hazol; Iliadin; Nafrine; Navasin; Navisin; Nezeril; Oximetazolina; Oximetazolinum; Oxylazine; Oxymetazolinum; Oxymethazoline; Oxymetozoline; Rhinofrenol; Rhinolitan; Sinerol; Dristan Long Lasting Mentholated Nasal Spray; Dristan Long Lasting Nasal Mist; Drixoral Nasal Solution; Oxymeta zoline; Oxymetazoline hydrochloride crystalline; Afrin Cherry 12 Hour Nasal Spray; Afrin Extra Moisturizing 12 Hour Nasal Spray; Afrin Original 12 Hour Nasal Spray; Afrin Original 12 Hour Nose Drops; Afrin Original 12 Hour Pump Mist; Afrin Sinus 12 Hour Nasal Spray; Duramist Plus Up To 12 Hour Nasal Decongestant Spray; Genasal Nasal Spray Up to 12 Hour Relief; H 990; Nasal Relief 12 Hour Nasal Spray; Nostrilla 12 Hour Nasal Decongestant; Vicks Sinex 12 Hour Nasal Spray; Vicks Sinex 12 Hour Ultra Fine Mist for Sinus Relief; Decongestant (TN); Neo-Synephrine 12 Hour Spray; Operil (TN); Oximetazolina [INN-Spanish]; Oxymetazoline (INN); Oxymetazoline [INN:BAN]; Oxymetazolinum [INN-Latin]; Visine L.R; Visine L.R.; Phenol, 6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethyl-(7CI,8CI); Phenol, 3-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethyl-(9CI); 2-(4-tert-Butyl-2,6-dimethyl-3-hydroxybenzyl)-2-imidazoline; 3-(4,5-dihydro-1H-imidazol-2-ylmethyl)-2,4-dimethyl-6-tert-butyl-phenol; 3-[(4,5-Dihydro-1H-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethylphenol; 6-t-Butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethylphenol; 6-tert-Butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethylphenol; 6-tert-Butyl-3-(4,5-dihydro-1H-imidazol-2-ylmethyl)-2,4-dimethylphenol
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Nasal Decongestants
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
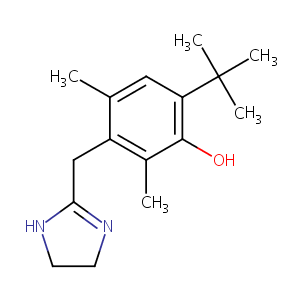 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 260.37 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Oxymetazoline (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Oxymetazoline FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| 3 | Tobias JD, Cartabuke R, Taghon T: Oxymetazoline (Afrin(R)): maybe there is more that we need to know. Paediatr Anaesth. 2014 Aug;24(8):795-8. doi: 10.1111/pan.12399. | ||||
| 4 | FDA Approved Drug Products: UPNEEQ (oxymetazoline hydrochloride ophthalmic solution), 0.1%, for topical ophthalmic use | ||||
| 5 | Jaillon P: Clinical pharmacokinetics of prazosin. Clin Pharmacokinet. 1980 Jul-Aug;5(4):365-76. doi: 10.2165/00003088-198005040-00004. | ||||
| 6 | Potent alpha(2A)-adrenoceptor-mediated vasoconstriction by brimonidine in porcine ciliary arteries. Invest Ophthalmol Vis Sci. 2001 Aug;42(9):2049-55. | ||||
| 7 | Identification and characterization of oxymetazoline glucuronidation in human liver microsomes: evidence for the involvement of UGT1A9. J Pharm Sci. 2011 Feb;100(2):784-93. | ||||
| 8 | Cell membrane chromatography competitive binding analysis for characterization of 1A adrenoreceptor binding interactions. Anal Bioanal Chem. 2011 Jul;400(10):3625-33. doi: 10.1007/s00216-011-5026-z. Epub 2011 May 5. | ||||
| 9 | Alpha-adrenoceptor agonistic activity of oxymetazoline and xylometazoline. Fundam Clin Pharmacol. 2010 Dec;24(6):729-39. | ||||
| 10 | Chromatography studies on bio-affinity of nine ligands of alpha1-adrenoceptor to alpha1D subtypes overexpressed in cell membrane. Sci China C Life Sci. 2004 Aug;47(4):376-81. doi: 10.1360/03yc0109. | ||||
| 11 | Cusson JR, Goldenberg E, Larochelle P "Effect of a novel monoamine-oxidase inhibitor, moclobemide on the sensitivity to intravenous tyramine and norepinephrine in humans." J Clin Pharmacol 31 (1991): 462-7. [PMID: 2050833] | ||||
| 12 | Product Information. Cymbalta (duloxetine). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 13 | Mendelson J, Jones RT, Upton R, Jacob P 3rd "Methamphetamine and ethanol interactions in humans." Clin Pharmacol Ther 57 (1995): 559-68. [PMID: 7768079] | ||||
| 14 | Barthel W, Glusa E, Koth W "Interactions of dihydroergotamine with etilefrine in human leg veins in vitro and in situ." Int J Clin Pharmacol Ther Toxicol 25 (1987): 63-9. [PMID: 2881898] | ||||
