Details of the Drug Combinations
General Information of This Drug (ID: DM92AH3)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ciproheptadina; Ciprovit; Cypoheptadine; Cyproheptadiene; Cyproheptadinum; Dihexazin; Dronactin; Eiproheptadine; Periactin; Periactine; Periactinol; Viternum; Cyproheptadine Hcl; MK 141; Ciproheptadina [INN-Spanish]; Ciprovit (TN); Cyproheptadine (INN); Cyproheptadine [INN:BAN]; Cyproheptadinum [INN-Latin]; Dibenzosuberonone/Cyproheptadine; Periactin (TN); 1-Methyl-4-(5-dibenzo(a,e)cycloheptatrienylidene)piperidine; 1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)piperidine; 4-(5-Dibenzo(a,d)cyclohepten-5-ylidine)-1-methylpiperidine; 4-(5H-Dibenzo(a,d)cyclohepten-5-ylidene)-1-methylpiperidene; 4-(5H-Dibenzo(a,d)cyclohepten-5-ylidene)-1-methylpiperidine; 4-(5H-Dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine; 4-(5H-dibenzo[a,d][7]annulen-5-ylidene)-1-methylpiperidine; 5-(1-Methylpiperidylidene-4)-5H-dibenzo(a,d)cyclopheptene
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihistamines
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
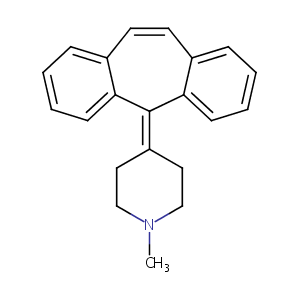 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
