Details of the Drug Combinations
General Information of This Drug (ID: DM9NJ52)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acecor; Fosenopril; Monopril; Staril; FOSINOPRIL SODIUM; Fosinopril (INN); Fosinopril [INN:BAN]; Monopril (TN); SQ-28555; SQ-28,555; Fosinopril, (1(S*(R*)),2 alpha,4 beta)-Isomer; (2S,4S)-4-cyclohexyl-1-[2-[(2-methyl-1-propanoyloxypropoxy)-(4-phenylbutyl)phosphoryl]acetyl]pyrrolidine-2-carboxylic acid; (2S,4S)-4-cyclohexyl-1-[2-[[(1S)-2-methyl-1-propanoyloxypropoxy]-(4-phenylbutyl)phosphoryl]acetyl]pyrrolidine-2-carboxylic acid; (2S,4S)-4-cyclohexyl-1-{2-[(2-methyl-1-propionyloxy-propoxy)-(4-phenyl-butyl)-phosphinoyl]-acetyl}-pyrrolidine-2-carboxylic acid; (4S)-4-cyclohexyl-1-({[2-methyl-1-(propanoyloxy)propoxy](4-phenylbutyl)phosphoryl}acetyl)-L-proline; (4S)-4-cyclohexyl-1-{[{[2-methyl-1-(propanoyloxy)propyl]oxy}(4-phenylbutyl)phosphoryl]acetyl}-L-proline; 4-cyclohexyl-1-[2-[(2-methyl-1-propanoyloxypropoxy)-(4-phenylbutyl)phosphoryl]acetyl]pyrrolidine-2-carboxylic acid
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
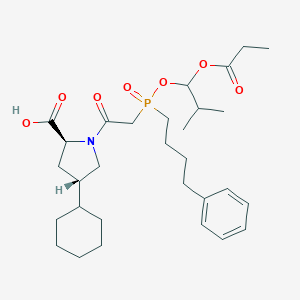 |
||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
