Details of the Drug Combinations
General Information of This Drug (ID: DM9T6MS)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CVT-510; 204512-90-3; UNII-GZ1X96601Z; CHEMBL392149; GZ1X96601Z; (2R,3S,4R,5R)-2-(hydroxymethyl)-5-[6-[[(3R)-oxolan-3-yl]amino]purin-9-yl]oxolane-3,4-diol; BDBM50224766; Tecadenoson [USAN:INN]; Tecadenoson (USAN/INN); AC1L4KMO; SCHEMBL246787; GTPL5592; CHEMBL356254; DTXSID80174415; BDBM50138530; DB04954; N6-[3-(R)-tetrahydrofuranyl]adenosine; HY-19661; Adenosine, N-(3R)-tetrahydro-3-furanyl-; LS-190860; Adenosine, N-(3R)-tetrahydro-3-furanyl)-; CS-0016174; D06019; CVT-510; N-(3-Tetrahydrofuranyl)-6-aminopurine riboside; Selenocompounds
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
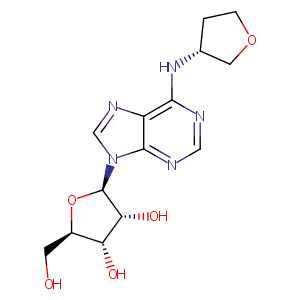 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
