Details of the Drug Combinations
General Information of This Drug (ID: DMBL3VD)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
PKI-587; 1197160-78-3; Gedatolisib; PF-05212384; PKI587; PKI 587; 1-(4-(4-(Dimethylamino)piperidine-1-carbonyl)phenyl)-3-(4-(4,6-dimorpholino-1,3,5-triazin-2-yl)phenyl)urea; PF 05212384; UNII-96265TNH2R; PF-05212384 (PKI-587); CHEMBL592445; 96265TNH2R; N-[4-[[4-(Dimethylamino)-1-piperidinyl]carbonyl]phenyl]-N'-[4-[4,6-di(4-morpholinyl)-1,3,5-triazin-2-yl]phenyl]urea; Gedatolisib (PF-05212384, PKI-587); Urea, N-(4-((4-(dimethylamino)-1-piperidinyl)carbonyl)phenyl)-N'-(4-(4,6-di-4-morpholinyl-1,3,5-triazin-2-yl)phenyl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
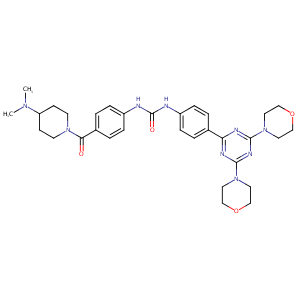 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
4 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
10 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
