Details of the Drug Combinations
General Information of This Drug (ID: DMBS3UG)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
relacorilant; Relacorilant; UNII-2158753C7E; 2158753C7E; 1496510-51-0; Relacorilant [INN]; Relacorilant [WHO-DD]; Relacorilant [USAN:INN]; SCHEMBL15454999; WANIDIGFXJFFEL-SANMLTNESA-N; CORT125134; ZINC141949519; AKOS032945759; J3.651.009I; ((4aR)-1-(4-fluorophenyl)-6-(1-methyl-1H-pyrazole-4-sulfonyl)-1,4,5,6,7,8-hexahydro-4aH-pyrazolo(3,4-g)isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methanone; Methanone, ((4aR)-1-(4-fluorophenyl)-1,4,5,6,7,8-hexahydro-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4ah-pyrazolo(3,4-g)isoqui
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Structure |
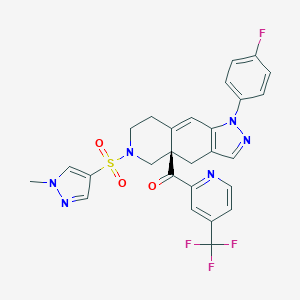 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
