Details of the Drug
General Information of Drug (ID: DMBS3UG)
| Drug Name |
CORT-125134
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
relacorilant; Relacorilant; UNII-2158753C7E; 2158753C7E; 1496510-51-0; Relacorilant [INN]; Relacorilant [WHO-DD]; Relacorilant [USAN:INN]; SCHEMBL15454999; WANIDIGFXJFFEL-SANMLTNESA-N; CORT125134; ZINC141949519; AKOS032945759; J3.651.009I; ((4aR)-1-(4-fluorophenyl)-6-(1-methyl-1H-pyrazole-4-sulfonyl)-1,4,5,6,7,8-hexahydro-4aH-pyrazolo(3,4-g)isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methanone; Methanone, ((4aR)-1-(4-fluorophenyl)-1,4,5,6,7,8-hexahydro-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4ah-pyrazolo(3,4-g)isoqui
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Structure |
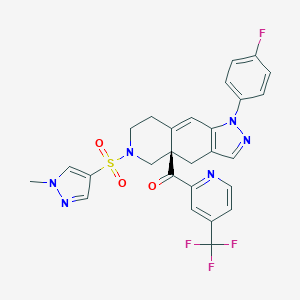 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 586.6 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
