Details of the Drug Combinations
General Information of This Drug (ID: DMC5SAW)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
LY3039478; Crenigacestat; 1421438-81-4; LY-3039478; UNII-923X28214S; 4,4,4-Trifluoro-N-((S)-1-(((S)-5-(2-hydroxyethyl)-6-oxo-6,7-dihydro-5H-benzo[d]pyrido[2,3-b]azepin-7-yl)amino)-1-oxopropan-2-yl)butanamide; 923X28214S; butanamide, n-[(1s)-2-[[(7s)-6,7-dihydro-5-(2-hydroxyethyl)-6-oxo-5h-pyrido[3,2-a][3]benzazepin-7-yl]amino]-1-methyl-2-oxoethyl]-4,4,4-trifluoro-; Butanamide, N-((1S)-2-(((7S)-6,7-dihydro-5-(2-hydroxyethyl)-6-oxo-5H-pyrido(3,2-a)(3)benzazepin-7-yl)amino)-1-methyl-2-oxoethyl)-4,4,4-trifluoro-
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
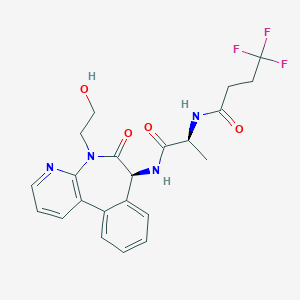 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
