Details of the Drug Combinations
General Information of This Drug (ID: DMCGATN)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
RVX 000222; 1044870-39-4; 2-(4-(2-Hydroxyethoxy)-3,5-dimethylphenyl)-5,7-dimethoxyquinazolin-4(3H)-one; RVX-000222; 2-[4-(2-Hydroxyethoxy)-3,5-Dimethylphenyl]-5,7-Dimethoxyquinazolin-4(3h)-One; UNII-8R4A7GDZ1D; RVX 000222; 8R4A7GDZ1D; QC-216; AK110565; Apabetalone [INN]; RVX000222; Apabetalone [USAN:INN]; 1K0; 4mr4; 4mr6; 4j1p; 4j3i; Apabetalone (USAN/INN); D0N7WW; SCHEMBL145019; GTPL7034; SCHEMBL17002023; AOB2133; DTXSID90146502; MolPort-027-835-445; NETXMUIMUZJUTB-UHFFFAOYSA-N; HMS3653C10; EX-A1110; BCP07787; ZINC4319
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Structure |
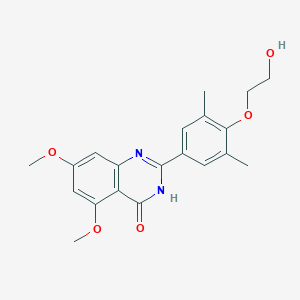 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
