Details of the Drug Combinations
General Information of This Drug (ID: DMCPF90)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ABBV-075; 1445993-26-9; UNII-VR86R11J7J; VR86R11J7J; N-[4-(2,4-Difluorophenoxy)-3-(6-Methyl-7-Oxo-6,7-Dihydro-1h-Pyrrolo[2,3-C]pyridin-4-Yl)phenyl]ethanesulfonamide; N-(4-(2,4-difluorophenoxy)-3-(6-methyl-7-oxo-6,7-dihydro-1H-pyrrolo[2,3-c]pyridin-4-yl)phenyl)ethanesulfonamide; 8NG; Mivebresib [INN]; ABBV-075 (Mivebresib); ABBV075; GTPL9117; SCHEMBL15068241; CHEMBL3987016; Mivebresib(ABBV-075 pound(c); MolPort-044-561-801; RDONXGFGWSSFMY-UHFFFAOYSA-N; BDBM220447; EX-A1082; s8400; ZINC146486516; AKOS030628486; CS-5815
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Structure |
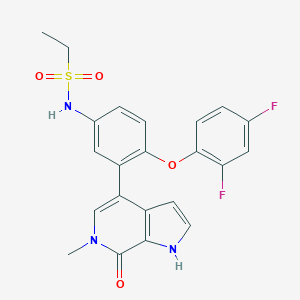 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
References
