Details of the Drug Combinations
General Information of This Drug (ID: DMCVJ86)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CAFdA; CFB; Clofarabina; Clofarabinum; Clofarex; Clolar; Evoltra; Clofarabine [USAN]; Clolar (TN); Evoltra (TN); Clofarabine (USAN/INN); Clolar, Evoltra, Clofarabine; Cl-F-Ara-A; (2R,3R,4S,5R)-5-(6-amino-2-chloropurin-9-yl)-4-fluoro-2-(hydroxymethyl)oxolan-3-ol; 2-Chloro-9-(2-deoxy-2-fluoro-b-D-arabinofuranosyl)-9H-purin-6-amine; 2-Chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)-9H-purin-6-amine; 2-Chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine; 2-Cl-2'-F-araA; 2-chloro-9-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)adenine; 3S211048
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
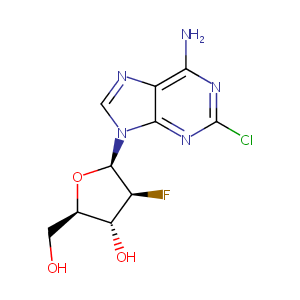 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
16 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
