Details of the Drug Combinations
General Information of This Drug (ID: DMD59GI)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
urapidil; 34661-75-1; Ebrantil; Eupressyl; Mediatensyl; Uraprene; Urapidilum [INN-Latin]; UNII-A78GF17HJS; NSC 310405; B-66256; C20H29N5O3; EINECS 252-130-4; BRN 0725782; A78GF17HJS; MLS003115907; 6-(3-(4-(o-Methoxyphenyl)-1-piperazinyl)propylamino)-1,3-dimethyluracil; 6-[[3-[4-(2-Methoxyphenyl)-1-piperazinyl]propyl]amino]-1,3-dimethyluracil; NCGC00016066-07; Ebrantil (TN); 6-((3-(4-(o-Methoxyphenyl)-1-piperazinyl)propyl)amino)-1,3-dimethyluracil; W-106718; Urapidilum; urapidil(jan); TgAAV-TNFR:Fc
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
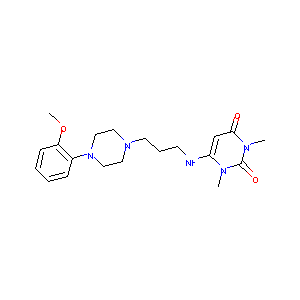 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
