Details of the Drug
General Information of Drug (ID: DMD59GI)
| Drug Name |
Urapidil
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
urapidil; 34661-75-1; Ebrantil; Eupressyl; Mediatensyl; Uraprene; Urapidilum [INN-Latin]; UNII-A78GF17HJS; NSC 310405; B-66256; C20H29N5O3; EINECS 252-130-4; BRN 0725782; A78GF17HJS; MLS003115907; 6-(3-(4-(o-Methoxyphenyl)-1-piperazinyl)propylamino)-1,3-dimethyluracil; 6-[[3-[4-(2-Methoxyphenyl)-1-piperazinyl]propyl]amino]-1,3-dimethyluracil; NCGC00016066-07; Ebrantil (TN); 6-((3-(4-(o-Methoxyphenyl)-1-piperazinyl)propyl)amino)-1,3-dimethyluracil; W-106718; Urapidilum; urapidil(jan); TgAAV-TNFR:Fc
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
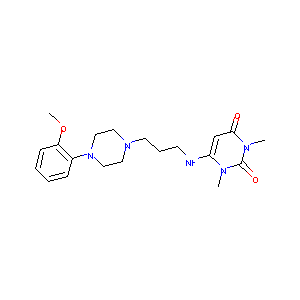 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 387.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.5 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hypertension | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA00-BA04 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
