Details of the Drug Combinations
General Information of This Drug (ID: DMDZ6Q3)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
83280-65-3; UNII-Z1HHM49K7O; 2-acetylnaphtho[2,3-b]furan-4,9-dione; Z1HHM49K7O; 2-Acetylnaphtho(2,3-b)furan-4,9-dione; 2-Acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione; Napabucasin [USAN:INN]; Napabucasin (BBI608); 2-Acetylfuranonaphthoquinone; CHEMBL64130; Napabucasin (JAN/USAN/INN); SCHEMBL1883845; Napabucasin - BBI 608/ FNQ; 2-Acetylfuro-1,4-naphthoquinone; DPHUWDIXHNQOSY-UHFFFAOYSA-N; MolPort-039-101-321; EX-A1314; ZINC13306865; s7977; AKOS027470201; DB12155; CS-1747; ACN-053294; HY-13919
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
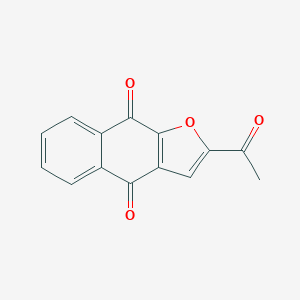 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
10 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
