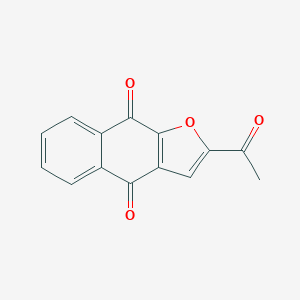
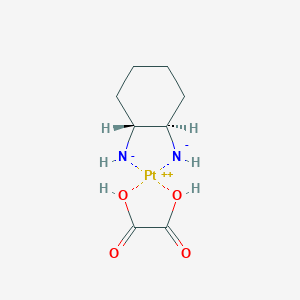
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
Serine/threonine-protein kinase Chk1 (CHEK1)
|
OTTTI622
|
CHK1_HUMAN
|
Decreases Expression
|
[7] |
|
Inhibitor of nuclear factor kappa-B kinase subunit beta (IKBKB)
|
OT9RDS3H
|
IKKB_HUMAN
|
Decreases Expression
|
[7] |
|
Baculoviral IAP repeat-containing protein 5 (BIRC5)
|
OTILXZYL
|
BIRC5_HUMAN
|
Decreases Expression
|
[8] |
|
Krueppel-like factor 4 (KLF4)
|
OT4O9RQW
|
KLF4_HUMAN
|
Decreases Expression
|
[8] |
|
Tyrosine-protein kinase JAK2 (JAK2)
|
OTBIDOOR
|
JAK2_HUMAN
|
Decreases Expression
|
[7] |
|
Serine/threonine-protein kinase LATS1 (LATS1)
|
OTOOCG4R
|
LATS1_HUMAN
|
Decreases Expression
|
[7] |
|
Aldehyde dehydrogenase 1A1 (ALDH1A1)
|
OTCUWZKB
|
AL1A1_HUMAN
|
Decreases Expression
|
[7] |
|
Hypoxanthine-guanine phosphoribosyltransferase (HPRT1)
|
OTOEEEXG
|
HPRT_HUMAN
|
Decreases Expression
|
[7] |
|
Myc proto-oncogene protein (MYC)
|
OTPV5LUK
|
MYC_HUMAN
|
Decreases Expression
|
[7] |
|
Pro-epidermal growth factor (EGF)
|
OTANRJ0L
|
EGF_HUMAN
|
Decreases Expression
|
[7] |
|
Receptor tyrosine-protein kinase erbB-2 (ERBB2)
|
OTOAUNCK
|
ERBB2_HUMAN
|
Decreases Expression
|
[7] |
|
Large ribosomal subunit protein uL10 (RPLP0)
|
OT3XKD6Y
|
RLA0_HUMAN
|
Decreases Expression
|
[7] |
|
Integrin beta-1 (ITGB1)
|
OT6G1IAR
|
ITB1_HUMAN
|
Decreases Expression
|
[7] |
|
Interleukin-8 (CXCL8)
|
OTS7T5VH
|
IL8_HUMAN
|
Decreases Expression
|
[7] |
|
Integrin alpha-4 (ITGA4)
|
OTSCAL0V
|
ITA4_HUMAN
|
Decreases Expression
|
[7] |
|
Electron transfer flavoprotein subunit alpha, mitochondrial (ETFA)
|
OTXX61VZ
|
ETFA_HUMAN
|
Decreases Expression
|
[7] |
|
Mucin-1 (MUC1)
|
OTHQI7IY
|
MUC1_HUMAN
|
Decreases Expression
|
[7] |
|
CD44 antigen (CD44)
|
OT9TTJ41
|
CD44_HUMAN
|
Decreases Expression
|
[7] |
|
Platelet endothelial cell adhesion molecule (PECAM1)
|
OTXOM4D9
|
PECA1_HUMAN
|
Decreases Expression
|
[7] |
|
Epithelial cell adhesion molecule (EPCAM)
|
OTHBZK5X
|
EPCAM_HUMAN
|
Decreases Expression
|
[7] |
|
Integrin alpha-2 (ITGA2)
|
OTPFL017
|
ITA2_HUMAN
|
Decreases Expression
|
[7] |
|
Endoglin (ENG)
|
OTL2LSMI
|
EGLN_HUMAN
|
Decreases Expression
|
[7] |
|
Nuclear factor NF-kappa-B p105 subunit (NFKB1)
|
OTNRRD8I
|
NFKB1_HUMAN
|
Decreases Expression
|
[7] |
|
Kit ligand (KITLG)
|
OTB9AVQ4
|
SCF_HUMAN
|
Decreases Expression
|
[7] |
|
Fibroblast growth factor receptor 2 (FGFR2)
|
OTLOPACK
|
FGFR2_HUMAN
|
Decreases Expression
|
[7] |
|
Integrin alpha-6 (ITGA6)
|
OT3FA39C
|
ITA6_HUMAN
|
Decreases Expression
|
[7] |
|
Signal transducer CD24 (CD24)
|
OT7CODJ4
|
CD24_HUMAN
|
Decreases Expression
|
[7] |
|
DNA cytosine-5)-methyltransferase 1 (DNMT1)
|
OTM2DGTK
|
DNMT1_HUMAN
|
Decreases Expression
|
[7] |
|
Hematopoietic progenitor cell antigen CD34 (CD34)
|
OT1MOFLZ
|
CD34_HUMAN
|
Decreases Expression
|
[7] |
|
Tyrosine-protein kinase receptor UFO (AXL)
|
OTKA2SUX
|
UFO_HUMAN
|
Decreases Expression
|
[7] |
|
Catenin beta-1 (CTNNB1)
|
OTZ932A3
|
CTNB1_HUMAN
|
Decreases Expression
|
[7] |
|
Polycomb complex protein BMI-1 (BMI1)
|
OTROVLJ0
|
BMI1_HUMAN
|
Decreases Expression
|
[7] |
|
TGF-beta receptor type-1 (TGFBR1)
|
OT40S1SJ
|
TGFR1_HUMAN
|
Decreases Expression
|
[7] |
|
DNA-binding protein inhibitor ID-1 (ID1)
|
OTKGNZN5
|
ID1_HUMAN
|
Decreases Expression
|
[7] |
|
Neurogenic locus notch homolog protein 1 (NOTCH1)
|
OTI1WADQ
|
NOTC1_HUMAN
|
Decreases Expression
|
[7] |
|
Transcription factor SOX-2 (SOX2)
|
OTFAWCAU
|
SOX2_HUMAN
|
Decreases Expression
|
[7] |
|
Glycogen synthase kinase-3 beta (GSK3B)
|
OTL3L14B
|
GSK3B_HUMAN
|
Decreases Expression
|
[7] |
|
Ataxin-1 (ATXN1)
|
OTQF0HNR
|
ATX1_HUMAN
|
Decreases Expression
|
[7] |
|
Actin, cytoplasmic 1 (ACTB)
|
OT1MCP2F
|
ACTB_HUMAN
|
Decreases Expression
|
[7] |
|
Beta-2-microglobulin (B2M)
|
OTDWN6NX
|
B2MG_HUMAN
|
Decreases Expression
|
[7] |
|
Protein jagged-1 (JAG1)
|
OT3LGT6K
|
JAG1_HUMAN
|
Decreases Expression
|
[7] |
|
POU domain, class 5, transcription factor 1 (POU5F1)
|
OTDHHN7O
|
PO5F1_HUMAN
|
Decreases Expression
|
[7] |
|
Urokinase plasminogen activator surface receptor (PLAUR)
|
OTIRKKEQ
|
UPAR_HUMAN
|
Decreases Expression
|
[7] |
|
Neurogenic locus notch homolog protein 2 (NOTCH2)
|
OTQ3Y9PA
|
NOTC2_HUMAN
|
Decreases Expression
|
[7] |
|
Epithelial discoidin domain-containing receptor 1 (DDR1)
|
OTYW1SH8
|
DDR1_HUMAN
|
Decreases Expression
|
[7] |
|
Tyrosine-protein kinase Mer (MERTK)
|
OTWBRAJ6
|
MERTK_HUMAN
|
Decreases Expression
|
[7] |
|
Serine-protein kinase ATM (ATM)
|
OTQVOHLT
|
ATM_HUMAN
|
Decreases Expression
|
[7] |
|
Histone deacetylase 1 (HDAC1)
|
OTQDNOXZ
|
HDAC1_HUMAN
|
Decreases Expression
|
[7] |
|
CD166 antigen (ALCAM)
|
OTCO4LP7
|
CD166_HUMAN
|
Decreases Expression
|
[7] |
|
Flotillin-2 (FLOT2)
|
OTZ0QR5L
|
FLOT2_HUMAN
|
Decreases Expression
|
[7] |
|
Tafazzin (TAFAZZIN)
|
OTDR57KI
|
TAZ_HUMAN
|
Decreases Expression
|
[7] |
|
Krueppel-like factor 17 (KLF17)
|
OT5NWVP7
|
KLF17_HUMAN
|
Decreases Expression
|
[7] |
|
Mastermind-like protein 1 (MAML1)
|
OTQA4DDN
|
MAML1_HUMAN
|
Decreases Expression
|
[7] |
|
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
|
OTAYZMOY
|
SIR1_HUMAN
|
Decreases Expression
|
[7] |
|
Protein smoothened (SMO)
|
OTXXE208
|
SMO_HUMAN
|
Decreases Expression
|
[7] |
|
Forkhead box protein P1 (FOXP1)
|
OTSG6XGF
|
FOXP1_HUMAN
|
Decreases Expression
|
[7] |
|
Homeobox protein NANOG (NANOG)
|
OTUEY1FM
|
NANOG_HUMAN
|
Decreases Expression
|
[7] |
|
Protein lin-28 homolog A (LIN28A)
|
OTJJBRCQ
|
LN28A_HUMAN
|
Decreases Expression
|
[7] |
|
Dachshund homolog 1 (DACH1)
|
OTMKNAGG
|
DACH1_HUMAN
|
Decreases Expression
|
[7] |
| ------------------------------------------------------------------------------------ |
|
|
|
|