Details of the Drug Combinations
General Information of This Drug (ID: DME6G97)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-21904A5386; 1061337-51-6; CHEMBL3291398; 21904A5386; Lefamulin [INN]; Acetic acid, 2-(((1R,2R,4R)-4-amino-2-hydroxycyclohexyl)thio)-, (3aS,4R,5S,6S,8R,9R,9aR,10R)-6-ethenyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3ah-cyclopentacycloocten-8-yl ester; Lefamulin(BC-3781); Lefamulin [USAN:INN]; Acetic acid, 2-[[(1R,2R,4R)-4-amino-2-hydroxycyclohexyl]thio]-, (3aS,4R,5S,6S,8R,9R,9aR,10R)-6-ethenyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3aH-cyclopentacycloocten
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Structure |
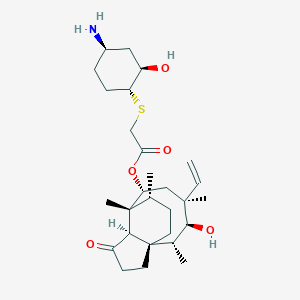 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
