Details of the Drug
General Information of Drug (ID: DME6G97)
| Drug Name |
Lefamulin
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-21904A5386; 1061337-51-6; CHEMBL3291398; 21904A5386; Lefamulin [INN]; Acetic acid, 2-(((1R,2R,4R)-4-amino-2-hydroxycyclohexyl)thio)-, (3aS,4R,5S,6S,8R,9R,9aR,10R)-6-ethenyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3ah-cyclopentacycloocten-8-yl ester; Lefamulin(BC-3781); Lefamulin [USAN:INN]; Acetic acid, 2-[[(1R,2R,4R)-4-amino-2-hydroxycyclohexyl]thio]-, (3aS,4R,5S,6S,8R,9R,9aR,10R)-6-ethenyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3aH-cyclopentacycloocten
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Structure |
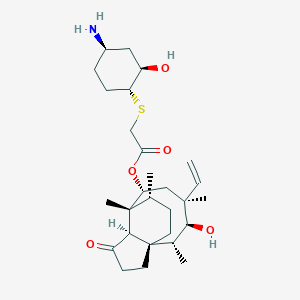 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 507.7 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Lefamulin (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2019 | ||||
|---|---|---|---|---|---|
| 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 3 | Wicha WW, Prince WT, Lell C, Heilmayer W, Gelone SP: Pharmacokinetics and tolerability of lefamulin following intravenous and oral dosing. J Antimicrob Chemother. 2019 Apr 1;74(Supplement_3):iii19-iii26. doi: 10.1093/jac/dkz087. | ||||
| 4 | Xenleta FDA label | ||||
| 5 | Amalakuhan B, Echevarria KL, Restrepo MI: Managing community acquired pneumonia in the elderly - the next generation of pharmacotherapy on the horizon. Expert Opin Pharmacother. 2017 Aug;18(11):1039-1048. doi: 10.1080/14656566.2017.1340937. Epub 2017 Jun 21. | ||||
| 6 | Molden E, Asberg A, Christensen H: Desacetyl-diltiazem displays severalfold higher affinity to CYP2D6 compared with CYP3A4. Drug Metab Dispos. 2002 Jan;30(1):1-3. | ||||
| 7 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 8 | Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy. 2018 Sep;38(9):935-946. | ||||
| 9 | Product Information. Fareston (toremifene). Schering Laboratories, Kenilworth, NJ. | ||||
| 10 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 11 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 12 | Patel S, Robinson R, Burk M "Hypertensive crisis associated with St. John's Wort." Am J Med 112 (2002): 507-8. [PMID: 11959071] | ||||
| 13 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
