Details of the Drug Combinations
General Information of This Drug (ID: DMF3AN7)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aerotina; Alarin; Alavert; Alerpriv; Allertidin; Anhissen; Biloina; Bonalerg; Civeran; Claratyne; Clarinase; Claritin; Claritine; Clarityn; Clarityne; Clarium; Fristamin; Histaloran; Lergy; Lertamine; Lesidas; Lisino; Loracert; Loradex; Loradif; Loranox; Lorantis; Lorastine; Loratadina; Loratadinum; Loratidine; Loratyne; Loraver; Lorfast; Loritine; Lowadina; Nularef; Optimin; Polaratyne; Pylor; Restamine; Rhinase; Rinomex; Roletra; Sanelor; Sensibit; Sohotin; Tadine; Velodan; Versal; Zeos; Bedix Loratadina; Claratyne Cold; Claratyne Decongestant; Clarinase Reperabs; Claritin D; Claritin Hives Relief; Claritin Hives Relief Reditab; Claritin Reditabs; Claritin reditab; Clarityne Dy Repetabs; Loratadina [Spanish]; Loratadine Redidose; Loratadine Wyeth Brand; Loratadinum [Latin]; Sinhistan Dy; Talorat Dy; Wyeth Brand of Loratadine; L 9664; Sch 29851; Sch29851; Alavert (TN); AllergyX (TN); Children's Claritin; Claritin (TN); Claritine (TN); Clarityn (TN); Clarityne (TN); Clarityne-D; Flonidan (TN); Fristamin (TN); Lomilan (TN); Lorfast (TN); Rinolan (TN); Roletra (TN); Sch-29851; Symphoral (TN); Tidilor (TN); Alavert, Claritin, Loratadine; Loratadine [USAN:BAN:INN]; Loratadine (JAN/USAN/INN); Ethyl 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidine-1-carboxylate; Ethyl 4-(8-chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-1-piperidinecarboxylate; 1-Piperidenecarboxylic acid, 4-(8-chloro-5,6-duhydro-11H-benzo [5,6]cyclohepta[1,2-b]-pyridin-11-ylidene)-, ethyl ester; 1-Piperidinecarboxylic acid, 4-(8-chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-, ethyl ester; 1-Piperidinecarboxylic acid, 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-, ethyl ester; 1-piperidinecarboxylic acid,4-(8-chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-,ethyl ester; 4-(8-Chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-1-piperidinecarboxylic Acid Ethyl Ester; 4-(8-Chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene-1-piperidinecarboxylic acidethyl ester; 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cycloheptal[1,2-b]pyridin-11-ylidene-1-piperidinecarboxylic acid ethyl ester
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihistamines
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
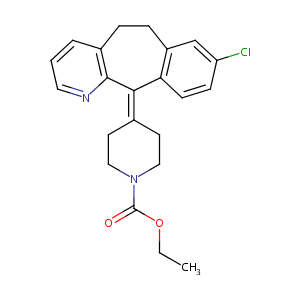 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
5 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||
References
