Details of the Drug
General Information of Drug (ID: DMF3AN7)
| Drug Name |
Loratadine
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aerotina; Alarin; Alavert; Alerpriv; Allertidin; Anhissen; Biloina; Bonalerg; Civeran; Claratyne; Clarinase; Claritin; Claritine; Clarityn; Clarityne; Clarium; Fristamin; Histaloran; Lergy; Lertamine; Lesidas; Lisino; Loracert; Loradex; Loradif; Loranox; Lorantis; Lorastine; Loratadina; Loratadinum; Loratidine; Loratyne; Loraver; Lorfast; Loritine; Lowadina; Nularef; Optimin; Polaratyne; Pylor; Restamine; Rhinase; Rinomex; Roletra; Sanelor; Sensibit; Sohotin; Tadine; Velodan; Versal; Zeos; Bedix Loratadina; Claratyne Cold; Claratyne Decongestant; Clarinase Reperabs; Claritin D; Claritin Hives Relief; Claritin Hives Relief Reditab; Claritin Reditabs; Claritin reditab; Clarityne Dy Repetabs; Loratadina [Spanish]; Loratadine Redidose; Loratadine Wyeth Brand; Loratadinum [Latin]; Sinhistan Dy; Talorat Dy; Wyeth Brand of Loratadine; L 9664; Sch 29851; Sch29851; Alavert (TN); AllergyX (TN); Children's Claritin; Claritin (TN); Claritine (TN); Clarityn (TN); Clarityne (TN); Clarityne-D; Flonidan (TN); Fristamin (TN); Lomilan (TN); Lorfast (TN); Rinolan (TN); Roletra (TN); Sch-29851; Symphoral (TN); Tidilor (TN); Alavert, Claritin, Loratadine; Loratadine [USAN:BAN:INN]; Loratadine (JAN/USAN/INN); Ethyl 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidine-1-carboxylate; Ethyl 4-(8-chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-1-piperidinecarboxylate; 1-Piperidenecarboxylic acid, 4-(8-chloro-5,6-duhydro-11H-benzo [5,6]cyclohepta[1,2-b]-pyridin-11-ylidene)-, ethyl ester; 1-Piperidinecarboxylic acid, 4-(8-chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-, ethyl ester; 1-Piperidinecarboxylic acid, 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-, ethyl ester; 1-piperidinecarboxylic acid,4-(8-chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-,ethyl ester; 4-(8-Chloro-5,6-dihydro-11H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-ylidene)-1-piperidinecarboxylic Acid Ethyl Ester; 4-(8-Chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene-1-piperidinecarboxylic acidethyl ester; 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cycloheptal[1,2-b]pyridin-11-ylidene-1-piperidinecarboxylic acid ethyl ester
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihistamines
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
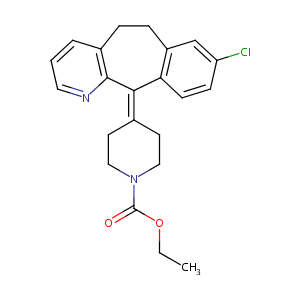 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 382.9 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.2 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Allergy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 4A80-4A85 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Loratadine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7216). | ||||
|---|---|---|---|---|---|
| 2 | Loratadine FDA Label | ||||
| 3 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015252) | ||||
| 4 | Loratadine: a nonsedating antihistamine with once-daily dosing. DICP. 1989 Jun;23(6):445-50. | ||||
| 5 | BDDCS applied to over 900 drugs | ||||
| 6 | Clissold SP, Sorkin EM, Goa KL: Loratadine. A preliminary review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1989 Jan;37(1):42-57. | ||||
| 7 | Health Canada Product Monograph: Claritin (loratadine) | ||||
| 8 | Mezincescu A, Karthikeyan VJ, Nadar SK: Ranolazine: A true pluripotent cardiovascular drug or jack of all trades, master of none? Sultan Qaboos Univ Med J. 2018 Feb;18(1):e13-e23. doi: 10.18295/squmj.2018.18.01.003. Epub 2018 Apr 4. | ||||
| 9 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 10 | Comparative Pharmacology of the H1 Antihistamines | ||||
| 11 | Clinical research of Ibudilast on treating the steroid resistant allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009 Jan;23(2):63-6. | ||||
| 12 | P-glycoprotein limits the brain penetration of nonsedating but not sedating H1-antagonists. Drug Metab Dispos. 2003 Mar;31(3):312-8. | ||||
| 13 | Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448. | ||||
| 14 | In vitro characterization of the inhibition profile of loratadine, desloratadine, and 3-OH-desloratadine for five human cytochrome P-450 enzymes. Drug Metab Dispos. 2001 Sep;29(9):1173-5. | ||||
| 15 | In vitro inhibition of human liver drug metabolizing enzymes by second generation antihistamines. Chem Biol Interact. 1999 Nov 15;123(1):63-79. | ||||
| 16 | Advances in high-resolution MS and hepatocyte models solve a long-standing metabolism challenge: the loratadine story. Bioanalysis. 2016 Aug;8(16):1645-62. | ||||
| 17 | Metabolism of loratadine and further characterization of its in vitro metabolites. Drug Metab Lett. 2009 Aug;3(3):162-70. | ||||
| 18 | Role of cytochrome P450 2C8 in drug metabolism and interactions. Pharmacol Rev. 2016 Jan;68(1):168-241. | ||||
| 19 | A toxicogenomic approach to drug-induced phospholipidosis: analysis of its induction mechanism and establishment of a novel in vitro screening system. Toxicol Sci. 2005 Feb;83(2):282-92. | ||||
| 20 | Testing of the inhibitory effects of loratadine and desloratadine on SARS-CoV-2 spike pseudotyped virus viropexis. Chem Biol Interact. 2021 Apr 1;338:109420. doi: 10.1016/j.cbi.2021.109420. Epub 2021 Feb 18. | ||||
| 21 | An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20. | ||||
| 22 | In vitro detection of drug-induced phospholipidosis using gene expression and fluorescent phospholipid based methodologies. Toxicol Sci. 2007 Sep;99(1):162-73. | ||||
| 23 | A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol Sci. 2013 Nov;136(1):216-41. | ||||
| 24 | H1-receptor antagonists terfenadine and loratadine inhibit spontaneous growth of neoplastic mast cells. Exp Hematol. 2010 Oct;38(10):896-907. doi: 10.1016/j.exphem.2010.05.008. Epub 2010 Jun 1. | ||||
| 25 | Palmitate increases the susceptibility of cells to drug-induced toxicity: an in vitro method to identify drugs with potential contraindications in patients with metabolic disease. Toxicol Sci. 2012 Oct;129(2):346-62. doi: 10.1093/toxsci/kfs208. Epub 2012 Jun 14. | ||||
| 26 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 27 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 28 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 29 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 30 | Ament PW, Paterson A "Drug interactions with the nonsedating antihistamines." Am Fam Physician 56 (1997): 223. [PMID: 9225677] | ||||
| 31 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 32 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 33 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 34 | Product Information. Serzone (nefazodone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 35 | Product Information. Trileptal (oxcarbazepine) Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 36 | Product Information. Claritin (loratadine). Schering Laboratories, Kenilworth, NJ. | ||||
| 37 | AbdelRahman SM, Gotschall RR, Kauffman RE, Leeder JS, Kearns GL "Investigation of terbinafine as a CYP2D6 inhibitor in vivo." Clin Pharmacol Ther 65 (1999): 465-72. [PMID: 10340911] | ||||
| 38 | Product Information. Victrelis (boceprevir). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 39 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 40 | Product Information. Priftin (rifapentine). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 41 | Product Information. Sustiva (efavirenz). DuPont Pharmaceuticals, Wilmington, DE. | ||||
| 42 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 43 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 44 | Product Information. Vaprisol (conivaptan). Cumberland Pharmaceuticals Inc, Nashville, TN. | ||||
| 45 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 46 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 47 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 48 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 49 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 50 | Product Information. Gleevec (imatinib mesylate). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 51 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 52 | Doherty MM, Charman WN "The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism?" Clin Pharmacokinet 41 (2002): 235-53. [PMID: 11978143] | ||||
| 53 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 54 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 55 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 56 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 57 | Product Information. Tracleer (bosentan). Acetelion Pharmaceuticals US, Inc, South San Francisco, CA. | ||||
| 58 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 59 | Product Information. Celebrex (celecoxib). Searle, Chicago, IL. | ||||
| 60 | DSouza DL, Levasseur LM, Nezamis J, Robbins DK, Simms L, Koch KM "Effect of alosetron on the pharmacokinetics of alprazolam." J Clin Pharmacol 41 (2001): 452-4. [PMID: 11304902] | ||||
| 61 | Atar S, Freedberg NA, Antonelli D, Rosenfeld T "Torsades de pointes and QT prolongation due to a combination of loratadine and amiodarone." Pacing Clin Electrophysiol 26 (2003): 785-6. [PMID: 12698686] | ||||
