Details of the Drug Combinations
General Information of This Drug (ID: DMFIS4D)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CDD-0102; CDD-0262; CDD-0264; CDD-102; MCD-386 Forte; MCD-386 Transderm; MCD-386CR; MCD-386 Forte/Transderm; Tetrahydropyrimidine muscarinic M1 agonists (Alzeimer's disease), Cognitive Pharmaceuticals; Tetrahydropyrimidine muscarinic M1 agonists (Alzeimer's disease), University of Toledo; Tetrahydropyrimidine muscarinicM1 agonists (Alzheimer's disease), Mithridion; MCD-386 (oral controlled release, CNS disorders), Mithridion; MCD-386 (high dose transdermal, Alzheimer's disease/schizophrenia), Mithridion; MCD-386 (high dose, Alzheimer's disease/schizophrenia), Mithridion; MCD-386 (transdermal, Alzheimer's disease/schizophrenia), Mithridion
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure | 3D Structure is Not Available |
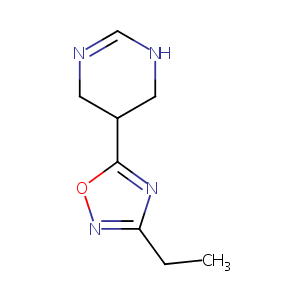 |
|||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
