Details of the Drug
General Information of Drug (ID: DMFIS4D)
| Drug Name |
MCD-386
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CDD-0102; CDD-0262; CDD-0264; CDD-102; MCD-386 Forte; MCD-386 Transderm; MCD-386CR; MCD-386 Forte/Transderm; Tetrahydropyrimidine muscarinic M1 agonists (Alzeimer's disease), Cognitive Pharmaceuticals; Tetrahydropyrimidine muscarinic M1 agonists (Alzeimer's disease), University of Toledo; Tetrahydropyrimidine muscarinicM1 agonists (Alzheimer's disease), Mithridion; MCD-386 (oral controlled release, CNS disorders), Mithridion; MCD-386 (high dose transdermal, Alzheimer's disease/schizophrenia), Mithridion; MCD-386 (high dose, Alzheimer's disease/schizophrenia), Mithridion; MCD-386 (transdermal, Alzheimer's disease/schizophrenia), Mithridion
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure | 3D Structure is Not Available |
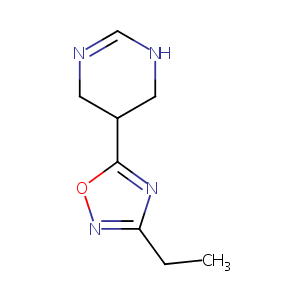 |
|||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Alzheimer disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A20 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
