Details of the Drug Combinations
General Information of This Drug (ID: DMFRM1I)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
842133-18-0; Invokana; Canagliflozin anhydrous; canagliflozin hemihydrate; UNII-6S49DGR869; JNJ-28431754; JNJ 24831754ZAE; Canagliflozin hydrate; TA-7284; 1-(Glucopyranosyl)-4-methyl-3-(5-(4-fluorophenyl)-2-thienylmethyl)benzene; CHEBI:73274; 6S49DGR869; (2S,3R,4R,5S,6R)-2-(3-((5-(4-FLUOROPHENYL)THIOPHEN-2-YL)METHYL)-4-METHYLPHENYL)-6-(HYDROXYMETHYL)TETRAHYDRO-2H-PYRAN-3,4,5-TRIOL; TA 7284; (1S)-1,5-anhydro-1-(3-{[5-(4-fluorophenyl)-2-thienyl]methyl}-4-methylphenyl)-D-glucitol; 928672-86-0
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
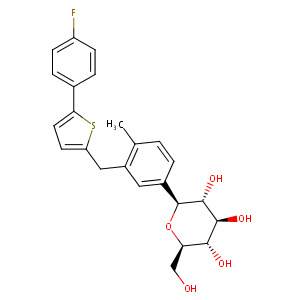 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
12 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
