Details of the Drug
General Information of Drug (ID: DMFRM1I)
| Drug Name |
Canagliflozin
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
842133-18-0; Invokana; Canagliflozin anhydrous; canagliflozin hemihydrate; UNII-6S49DGR869; JNJ-28431754; JNJ 24831754ZAE; Canagliflozin hydrate; TA-7284; 1-(Glucopyranosyl)-4-methyl-3-(5-(4-fluorophenyl)-2-thienylmethyl)benzene; CHEBI:73274; 6S49DGR869; (2S,3R,4R,5S,6R)-2-(3-((5-(4-FLUOROPHENYL)THIOPHEN-2-YL)METHYL)-4-METHYLPHENYL)-6-(HYDROXYMETHYL)TETRAHYDRO-2H-PYRAN-3,4,5-TRIOL; TA 7284; (1S)-1,5-anhydro-1-(3-{[5-(4-fluorophenyl)-2-thienyl]methyl}-4-methylphenyl)-D-glucitol; 928672-86-0
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
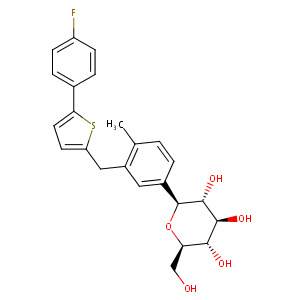 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 444.5 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.2 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Canagliflozin (Comorbidity)
|
|||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Canagliflozin FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4582). | ||||
| 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 5 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. 2017 Sep 6;11:2643-2651. | ||||
| 8 | In vitro and physiologically-based pharmacokinetic based assessment of drug-drug interaction potential of canagliflozin. Br J Clin Pharmacol. 2017 May;83(5):1082-1096. | ||||
| 9 | Sodium-glucose linked transporter 2 (SGLT2) inhibitors in the management of type-2 diabetes: a drug class overview. P T. 2015 Jul;40(7):451-62. | ||||
| 10 | Effects of rifampin, cyclosporine A, and probenecid on the pharmacokinetic profile of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in healthy participants. Int J Clin Pharmacol Ther. 2015 Feb;53(2):115-28. | ||||
| 11 | Discovery of novel N--D-xylosylindole derivatives as sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors for the management of hyperglycemia in diabetes. J Med Chem. 2011 Jan 13;54(1):166-78. doi: 10.1021/jm101072y. Epub 2010 Dec 3. | ||||
| 12 | Evaluation of inhibitors of intestinal UDP-glucuronosyltransferases 1A8 and 1A10 using raloxifene as a substrate in Caco-2 cells: Studies with four flavonoids of Scutellaria baicalensis. Toxicol In Vitro. 2021 Apr;72:105087. doi: 10.1016/j.tiv.2021.105087. Epub 2021 Jan 10. | ||||
| 13 | Cerner Multum, Inc. "Australian Product Information.". | ||||
