Details of the Drug Combinations
General Information of This Drug (ID: DMGFPU2)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Linezlid; ZLD; Zyvox; Zyvoxa; Zyvoxam; Zyvoxid; PNU 100766; U 100766; Linezolid & VRC3375; Linezolid [USAN:INN]; PNU-100766; U-100766; Zyvox (TN); Zyvoxam (TN); Zyvoxid (TN); NDA 21-130 Zyvox (linezolid tablets); NDA 21-131 Zyvox for injection (linezolid injection); NDA 21-132 Zyvox oral suspension (linzolid oral suspension); Linezolid (JAN/USAN/INN); PNU-100766, U-100766, Zyvoxid, Zyvoxam, Linezolid; N-((3-(3-fluoro-4-morpholinylphenyl)-2-oxo-5-oxazolidinyl)methyl)acetamide; N-(((S)-3-(3-Fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl)methyl)acetamide; N-[[(5S)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide; N-({5S)-3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-1,3-oxazolidin-5-yl}methyl)acetamide, N-[[(S)-3-(3-Fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl]acetamide; (S)-N-[[3-(3-fluoro-4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]methyl]acetamide; 111GE017
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
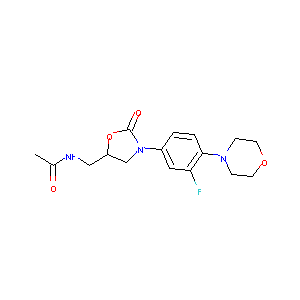 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
21 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
