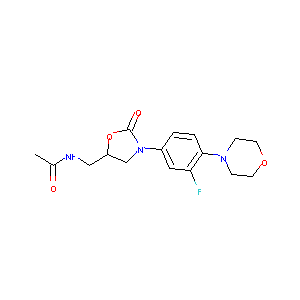
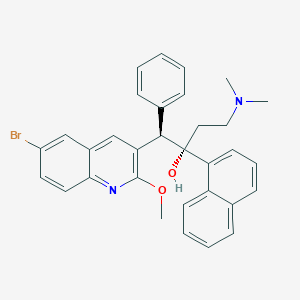
| 1 |
ClinicalTrials.gov (NCT02589782) Pragmatic Clinical Trial for a More Effective Concise and Less Toxic MDR-TB Treatment Regimen(s)
|
| 2 |
Linezolid FDA Label
|
| 3 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 200222.
|
| 4 |
Therapeutic and Triage Strategies for 2019 Novel Coronavirus Disease in Fever Clinics. Lancet Respir Med. 2020 Mar;8(3):e11-e12.
|
| 5 |
Bedaquiline FDA Label
|
| 6 |
Nat Rev Drug Discov. 2013 Feb;12(2):87-90.
|
| 7 |
Genome sequencing of linezolid-resistant Streptococcus pneumoniae mutants reveals novel mechanisms of resistance. Genome Res. 2009 Jul;19(7):1214-23.
|
| 8 |
Nonclinical Evaluation of Antibacterial Oxazolidinones Contezolid and Contezolid Acefosamil with Low Serotonergic Neurotoxicity. Chem Res Toxicol. 2021 May 17;34(5):1348-1354. doi: 10.1021/acs.chemrestox.0c00524. Epub 2021 Apr 29.
|
| 9 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 10 |
Bedaquiline metabolism: enzymes and novel metabolites. Drug Metab Dispos. 2014 May;42(5):863-6.
|
| 11 |
ClinicalTrials.gov (NCT02754765) Evaluating Newly Approved Drugs for Multidrug-resistant TB
|
| 12 |
ClinicalTrials.gov (NCT02333799) A Phase 3 Study Assessing the Safety and Efficacy of Bedaquiline Plus PA-824 Plus Linezolid in Subjects With Drug Resistant Pulmonary Tuberculosis
|
| 13 |
ClinicalTrials.gov (NCT04207112) Economic Evaluation of New MDR TB Regimens
|
|
|
|
|
|
|