Details of the Drug Combinations
General Information of This Drug (ID: DMH2NMY)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Carbocaine; Mepivacaina; Mepivacainum; Mepivicaine; Scandicain; Scandicaine; Scandicane; Arestocaine HCL; Carboplyin Dental; Isocaine HCL; Carbocaine (TN); Carboplyin Dental (TN); D-mepivacaine; DL-Mepivacaine; Mepivacaina [INN-Spanish]; Mepivacaine (INN); Mepivacaine [INN:BAN]; Mepivacainum [INN-Latin]; Polocaine (TN); Polocaine-Mpf; S-Ropivacaine Mesylate; D(-)-Mepivacaine; N-Methyl-2-pipecolic acid, 2,6-dimethylanilide; N-Methyl-2-pipecolic acid, 2,6-xylidide; N-Methylhexahydro-2-picolinic acid, 2,6-dimethylanilide; N-(2,6-Dimethylphenyl)-1-methyl-2-piperidinecarboxamide; N-(2,6-Dimethylphenyl)-1-methylpiperidine-2-carboxamide; (+-)-1-Methyl-2',6'-pipecoloxylidide; 1-METHYL-2',6'-PIPECOLOXYLIDIDE
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anesthetics
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
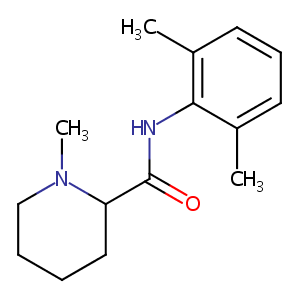 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
