Details of the Drug Combinations
General Information of This Drug (ID: DMHQV6R)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
tirabrutinib; Tirabrutinib; 1351636-18-4; UNII-LXG44NDL2T; LXG44NDL2T; Tirabrutinib [INN]; ONO-4059(Free base); SCHEMBL14798454; MolPort-044-728-902; BDBM194087; ZINC72318699; AKOS030526437; CS-5676; HY-15771; US9199997, 9; F10085; (R)-6-amino-9-(1-(but-2-ynoyl)pyrrolidin-3-yl)-7-(4-phenoxyphenyl)-7H-purin-8(9H)-one; 6-Amino-9-((3R)-1-(2-butynoyl)-3-pyrrolidinyl)-7-(4-phenoxyphenyl)-7,9-dihydro-8H-purin-8-one; 8H-Purin-8-one,6-amino-7,9-dihydro-9-((3R)-1-(1-oxo-2-butyn-1-yl)-3-pyrrolidinyl)-7-(4-phenoxyphenyl); 6-Amino-9-[(3R)-1-(2-butyno
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Structure |
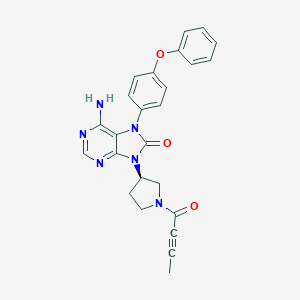 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
