Details of the Drug Combinations
General Information of This Drug (ID: DMHWE7P)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ABPC; Acillin; Adobacillin; Alpen; Amblosin; Amcill; Amfipen; Aminobenzylpenicillin; Ampen; Ampichel; Ampicil; Ampicilina; Ampicillanyl; Ampicillina; Ampicilline; Ampicillinum; Ampicin; Ampifarm; Ampikel; Ampimed; Ampipenin; Ampiscel; Ampisyn; Ampivax; Ampivet; Amplacilina; Amplin; Amplipenyl; Amplisom; Amplital; Austrapen; Binotal; Bonapicillin; Britacil; Campicillin; Cimex; Copharcilin; Delcillin; Deripen; Divercillin; Doktacillin; Duphacillin; Grampenil; Guicitrina; Guicitrine; Lifeampil; Morepen; Norobrittin; Nuvapen; Omnipen; Orbicilina; Penbristol; Penbritin; Penbrock; Penicline; Penimic; Pensyn; Pentrex; Pentrexl; Pentrexyl; Polycillin; Ponecil; Princillin; Principen; QIDamp; Racenacillin; Rosampline;Roscillin; Semicillin; Servicillin; Sumipanto; Supen; Synpenin; Texcillin; Tokiocillin; Tolomol; Totacillin; Totalciclina; Totapen; Trifacilina; Ukapen; Ultrabion; Ultrabron; Vampen; Viccillin; Wypicil; Amfipen V; Amipenix S; Ampicillin A; Ampicillin Anhydrous; Ampicillin Base; Ampicillin acid; Ampicillin anhydrate; Ampicillina [DCIT]; Anhydrous ampicillin; Olin Kid; Pen A; Pen A Oral; Pen Ampil;Penbritin paediatric; Penbritin syrup; Pfizerpen A; Semicillin R; Viccillin S; AY 6108; BA 7305; BRL 1341; Bayer 5427; HI 63; P 50; Principen 125; Principen 250; Principen 500; SQ 17382; AB-PC; AB-PCSol; AY-6108; Ambidrin (TN); Ampi-Co; Ampi-Tab; Ampi-bol; Ampicilina [INN-Spanish]; Ampicilline [INN-French]; Ampicillinum [INN-Latin]; Ampipenin, nt3; Ampy-Penyl; Anhydrous ampicillin (JP15); BRL-1341; D-Ampicillin; D-Cillin; KS-R1; Novo-ampicillin; OMNIPEN (AMPICILLIN); Omnipen (TN); Omnipen-N; P-50; Penbritin-S; Penicillin, Aminobenzyl; Pfizerpen-A; Polycillin-N; Polyflex (Veterinary); Ro-Ampen; SK-Ampicillin; Totacillin (sodium); Totacillin-N; WY-5103; Ampicillin (USP/INN); AMPICILLIN (SEE ALSO AMPICILLIN TRIHYDRATE 7177-48-2); Ampicillin [USAN:BAN:INN:JAN]; Ampicillin [USAN:INN:BAN:JAN];D-(-)-Ampicillin; D-(-)-alpha-Aminobenzylpenicillin; D-(-)-alpha-Aminopenicillin; D-(-)-6-(alpha-Aminophenylacetamido)penicillanic acid; 6-(D(-)-alpha-Aminophenylacetamido)penicillanic acid; 6beta-[(2R)-2-amino-2-phenylacetamido]-2,2-dimethylpenam-3alpha-carbonyl; 6beta-[(2R)-2-amino-2-phenylacetamido]-2,2-dimethylpenam-3alpha-carboxylic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
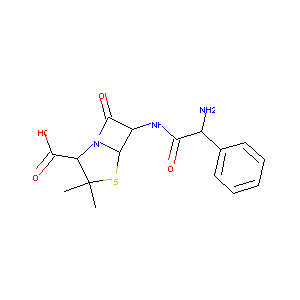 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
9 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
