Details of the Drug
General Information of Drug (ID: DMHWE7P)
| Drug Name |
Ampicillin
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ABPC; Acillin; Adobacillin; Alpen; Amblosin; Amcill; Amfipen; Aminobenzylpenicillin; Ampen; Ampichel; Ampicil; Ampicilina; Ampicillanyl; Ampicillina; Ampicilline; Ampicillinum; Ampicin; Ampifarm; Ampikel; Ampimed; Ampipenin; Ampiscel; Ampisyn; Ampivax; Ampivet; Amplacilina; Amplin; Amplipenyl; Amplisom; Amplital; Austrapen; Binotal; Bonapicillin; Britacil; Campicillin; Cimex; Copharcilin; Delcillin; Deripen; Divercillin; Doktacillin; Duphacillin; Grampenil; Guicitrina; Guicitrine; Lifeampil; Morepen; Norobrittin; Nuvapen; Omnipen; Orbicilina; Penbristol; Penbritin; Penbrock; Penicline; Penimic; Pensyn; Pentrex; Pentrexl; Pentrexyl; Polycillin; Ponecil; Princillin; Principen; QIDamp; Racenacillin; Rosampline;Roscillin; Semicillin; Servicillin; Sumipanto; Supen; Synpenin; Texcillin; Tokiocillin; Tolomol; Totacillin; Totalciclina; Totapen; Trifacilina; Ukapen; Ultrabion; Ultrabron; Vampen; Viccillin; Wypicil; Amfipen V; Amipenix S; Ampicillin A; Ampicillin Anhydrous; Ampicillin Base; Ampicillin acid; Ampicillin anhydrate; Ampicillina [DCIT]; Anhydrous ampicillin; Olin Kid; Pen A; Pen A Oral; Pen Ampil;Penbritin paediatric; Penbritin syrup; Pfizerpen A; Semicillin R; Viccillin S; AY 6108; BA 7305; BRL 1341; Bayer 5427; HI 63; P 50; Principen 125; Principen 250; Principen 500; SQ 17382; AB-PC; AB-PCSol; AY-6108; Ambidrin (TN); Ampi-Co; Ampi-Tab; Ampi-bol; Ampicilina [INN-Spanish]; Ampicilline [INN-French]; Ampicillinum [INN-Latin]; Ampipenin, nt3; Ampy-Penyl; Anhydrous ampicillin (JP15); BRL-1341; D-Ampicillin; D-Cillin; KS-R1; Novo-ampicillin; OMNIPEN (AMPICILLIN); Omnipen (TN); Omnipen-N; P-50; Penbritin-S; Penicillin, Aminobenzyl; Pfizerpen-A; Polycillin-N; Polyflex (Veterinary); Ro-Ampen; SK-Ampicillin; Totacillin (sodium); Totacillin-N; WY-5103; Ampicillin (USP/INN); AMPICILLIN (SEE ALSO AMPICILLIN TRIHYDRATE 7177-48-2); Ampicillin [USAN:BAN:INN:JAN]; Ampicillin [USAN:INN:BAN:JAN];D-(-)-Ampicillin; D-(-)-alpha-Aminobenzylpenicillin; D-(-)-alpha-Aminopenicillin; D-(-)-6-(alpha-Aminophenylacetamido)penicillanic acid; 6-(D(-)-alpha-Aminophenylacetamido)penicillanic acid; 6beta-[(2R)-2-amino-2-phenylacetamido]-2,2-dimethylpenam-3alpha-carbonyl; 6beta-[(2R)-2-amino-2-phenylacetamido]-2,2-dimethylpenam-3alpha-carboxylic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteriaListeria monocytogenesHaemophilus influenzaeGram-positive BacteriaStreptococcus pneumoniaeStreptococcus pyogenesGram-negative BacteriaNeisseria meningitidis
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
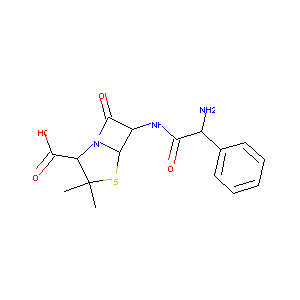 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 349.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Ampicillin (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Ampicillin FDA Label | ||||
|---|---|---|---|---|---|
| 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 061936. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Effects of amino acid alterations in penicillin-binding proteins (PBPs) 1a, 2b, and 2x on PBP affinities of penicillin, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor in 18 clinical isolates of penicillin-susceptible, -intermediate, and -resistant pneumococci. Antimicrob Agents Chemother. 2002 May;46(5):1273-80. | ||||
| 8 | Transport characteristics of a novel peptide transporter 1 substrate, antihypotensive drug midodrine, and its amino acid derivatives. J Pharmacol Exp Ther. 2006 Jul;318(1):455-60. | ||||
| 9 | Several hPepT1-transported drugs are substrates of the Escherichia coli proton-coupled oligopeptide transporter YdgR. Res Microbiol. 2017 Jun;168(5):443-449. | ||||
| 10 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | ||||
| 11 | A novel quorum-quenching N-acylhomoserine lactone acylase from Acidovorax sp. strain MR-S7 mediates antibiotic resistance. Appl Environ Microbiol. 2017 Jun 16;83(13). pii: e00080-17. | ||||
| 12 | Purification and characterization of a new beta-lactamase from Bacteroides uniformis. Antimicrob Agents Chemother. 1995 Jul;39(7):1458-61. | ||||
| 13 | Beta-lactamase production and antimicrobial susceptibility of subgingival bacteria from refractory periodontitis. Oral Microbiol Immunol. 2004 Oct;19(5):303-8. | ||||
| 14 | Drug-resistant genes carried by Acinetobacter baumanii isolated from patients with lower respiratory tract infection. Chin Med J (Engl). 2010 Sep;123(18):2571-5. | ||||
| 15 | Characterization of the AmpC beta-Lactamase from Burkholderia multivorans. Antimicrob Agents Chemother. 2018 Sep 24;62(10). pii: e01140-18. | ||||
| 16 | Beta-lactamase expression in Plesiomonas shigelloides. J Antimicrob Chemother. 2000 Jun;45(6):877-80. | ||||
| 17 | Comparison of phenotypic and transcriptomic effects of false-positive genotoxins, true genotoxins and non-genotoxins using HepG2 cells. Mutagenesis. 2011 Sep;26(5):593-604. | ||||
| 18 | Brause BD, Romankiewicz JA, Gotz V, Franklin JE Jr, Roberts RB "Comparative study of diarrhea associated with clindamycin and ampicillin therapy." Am J Gastroenterol 73 (1980): 244-8. [PMID: 7405925] | ||||
| 19 | Alexander DP, Russo ME, Fohrman DE, Rothstein G "Nafcillin-induced platelet dysfunction and bleeding." Antimicrob Agents Chemother 23 (1983): 59-62. [PMID: 6830209] | ||||
| 20 | Product Information. CellCept (mycophenolate mofetil). Roche Laboratories, Nutley, NJ. | ||||
| 21 | Product Information. Kapidex (dexlansoprazole). Takeda Pharmaceuticals America, Lincolnshire, IL. | ||||
| 22 | Allen MB, Fitzpatrick RW, Barratt A, Cole RB "The use of probenecid to increase the serum amoxycillin levels in patients with bronchiectasis." Respir Med 84 (1990): 143-6. [PMID: 2371437] | ||||
| 23 | Kwon OC, Lee JS, Kim YG, Lee CK, Yoo B, Hong S. Safety of the concomitant use of methotrexate and a prophylactic dose of trimethoprim-sulfamethoxazole.?Clin Rheumatol. 2018;37(12):3215-3220. [PMID: 29383453] | ||||
